Playlist
Show Playlist
Hide Playlist
Bohr Effect
-
Slides Hemoglobin Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
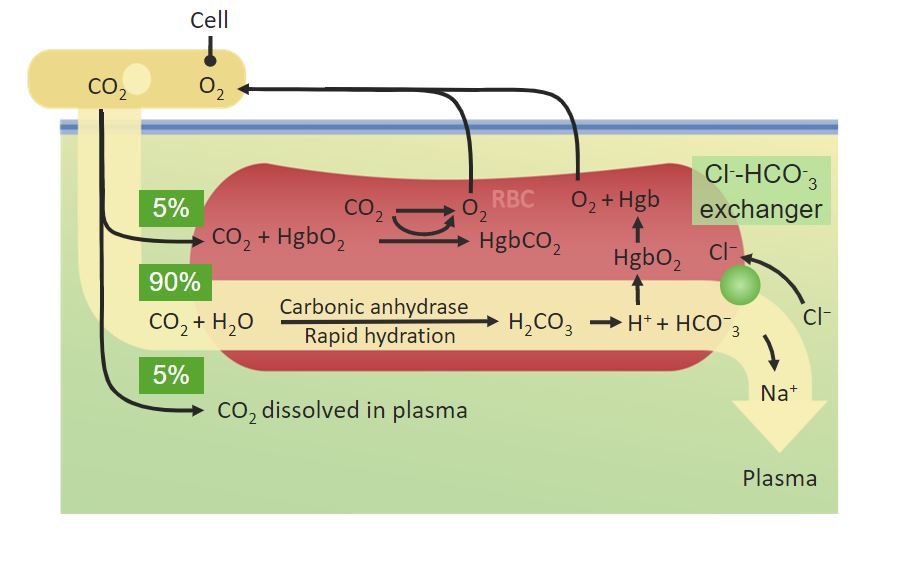
00:02 Now if we just think about hemoglobin for a second and we compare the oxygen binding properties of hemoglobin, we see something really interesting. 00:12 This plot shows four different measures of hemoglobin's ability to bind oxygen. 00:20 In each case, the only difference is the pH of the solution that the hemoglobin is found in. 00:27 The curve farthest on the left corresponds to hemoglobin binding oxygen at a pH of 7.6. 00:34 The next curve is at 7.4. 00:36 The next curve is at 7.2. 00:38 And the last curve, the farthest to the right is measuring hemoglobin's binding of oxygen at 7.0. 00:45 Now if we use that principle that I showed you of moving the curve to the right, when that happens, we're seeing less binding of oxygen, what this curve is what these curves are telling us, is that the lower the pH, the less hemoglobin will bind to oxygen. 01:02 Well, why is that important? The reason that that's important is cells that are rapidly metabolizing -- let's say that muscle cell that's out there while you're running that race. 01:11 Cells that ara rapidly metabolizing release protons. 01:16 And protons are what cause the pH to fall. 01:19 So that change in pH occurs around rapidly metabolizing cells. 01:24 And what cells need the most oxygen? The ones that are rapidly metabolizing. 01:30 So as we look at this graph, we see that if we look at a given oxygen concentration, we have less bound for any of the ones that have the lower pH. 01:40 And if we look at a given oxygen saturation, we see that it takes more oxygen to get to the same saturation level for the one with the lower pH. 01:48 Again, consistent with what I said earlier about the curve moving to the left having increased affinity for oxygen. 01:58 Now we learn something as a result to this graph. 02:02 And that is that protons are important in controlling hemoglobin's affinity for oxygen. 02:08 Well, this effect that I've just described to you is known as the Bohr Effect named for the person who discovered it at the turn of the 20th century. 02:15 Protons are important in this process because they actually work by binding to hemoglobin. 02:22 And when they bind to hemoglobin, they cause hemoglobin to have a slight change of shape. 02:27 We've seen numerous times now that changes of shape change the properties of the protein whose shape is being changed. 02:35 The reshape hemoglobin is losing some of its oxygen because it's less able to hold on to it. 02:42 And that shape changes happen because the protons are bound to it. 02:46 So since rapidly metabolizing tissues are releasing protons, the rapidly metabolizing tissues are getting more oxygen from hemoglobin. 02:55 Well, what I've shown you already is pretty remarkable about hemoglobin. 02:58 We see that it has changing affinity for oxygen depending upon the oxygen concentration and it has changing affinity for oxygen based on the concentration of the protons and the solution that it's in. 03:09 But that's not the only thing that hemoglobin is also interesting for. 03:12 This graph shows an addition to what I showed you in the last graph. 03:16 So let's step through it. 03:18 In this graph we see, in this set of graphs, we see three different solutions that hemoglobin is found in. 03:26 The first solution is the blue one. 03:28 That blue curve is the farthest to the left indicating the greatest affinity for oxygen. 03:34 That solution measures hemoglobin's affinity for oxygen at pH 7.4. 03:39 That’s about the normal pH of blood. 03:42 That's measured in the absence of carbon dioxide and I'll mention why that's important in a second. 03:48 The orange curve, the one in the middle is measured at pH of 7.2. 03:52 And consistent with what I showed you in the last graph, the affinity has shifted, meaning that this pH is causing hemoglobin to have less affinity for oxygen than the one at 7.4. 04:05 That's consistent with what we said before. 04:07 More protons, lower pH, lower pH, less affinity for oxygen. 04:12 But now, what's interesting here is if we look at the same pH of 7.2 but we add carbon dioxide to it, we see that the green curve has shifted further to the right. 04:23 What is that mean? It means that carbon dioxide can also affect hemoglobin's affinity for oxygen. 04:31 It causes hemoglobin to let go of its oxygen much more readily. 04:36 Now, so we now see that there are three things that can affect hemoglobin's affinity for oxygen, the concentration of oxygen, the concentration of protons and the concentration of carbon dioxide. 04:48 Why is carbon dioxide important? Well, carbon dioxide is the end product of respiration of cells. 04:55 Rapidly metabolizing cells in addition to releasing protons are releasing carbon dioxide. 05:02 And carbon dioxide is another -- carbon dioxide is another signal that the cell is saying, "Hey, I need the oxygen." And the oxygen is being left in the right place for it. 05:12 So we've seen now that acid favors the release of oxygen from hemoglobin. 05:16 Carbon dioxide favors the release of oxygen from hemoglobin. 05:20 And acid and carbon dioxide are released by rapidly metabolizing tissues. 05:24 We're starting to see a pattern.
About the Lecture
The lecture Bohr Effect by Kevin Ahern, PhD is from the course Amino Acid Metabolism.
Included Quiz Questions
In the Bohr effect, which of the following is true of hemoglobin?
- It has a greater affinity for oxygen at higher pH.
- It releases carbon dioxide when it binds to protons.
- It lets go of oxygen as the proton concentration decreases.
- It has a greater affinity for oxygen at lower pH.
- Protons have no effect on hemoglobin's shape.
In the Bohr effect, hemoglobin releases which of the following?
- Additional oxygen near rapidly metabolizing tissues
- Additional carbon dioxide in the presence of oxygen.
- Additional oxygen in the presence of oxygen and carbon dioxide
- Additional oxygen in the presence of oxygen
- Additional carbon dioxide in the presence of protons
Which of the following do not affect hemoglobin’s affinity for oxygen? (Check all that apply)
- Nitrogen concentration
- Na+ ion concentration
- Proton concentration
- Oxygen concentration
- Carbon dioxide concentration
How does the proton concentration affect the affinity of hemoglobin for oxygen?
- Protons bind to hemoglobin, lowering the affinity for oxygen.
- Protons change the amino acid sequence of hemoglobin, lowering its affinity for oxygen.
- Protons bind to hemoglobin, increasing its affinity for oxygen.
- Protons change the amino acid sequence of hemoglobin, increasing its affinity for oxygen.
- Protons allow more carbon dioxide in the blood, increasing hemoglobin's affinity for oxygen,
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Excellent lecture. Understood Bohr effect better than ever. I love these lectures.