Playlist
Show Playlist
Hide Playlist
Binding of Multiple Substrates – Enzyme Classification
-
02 Advanced Enzymes&Kinetics2.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
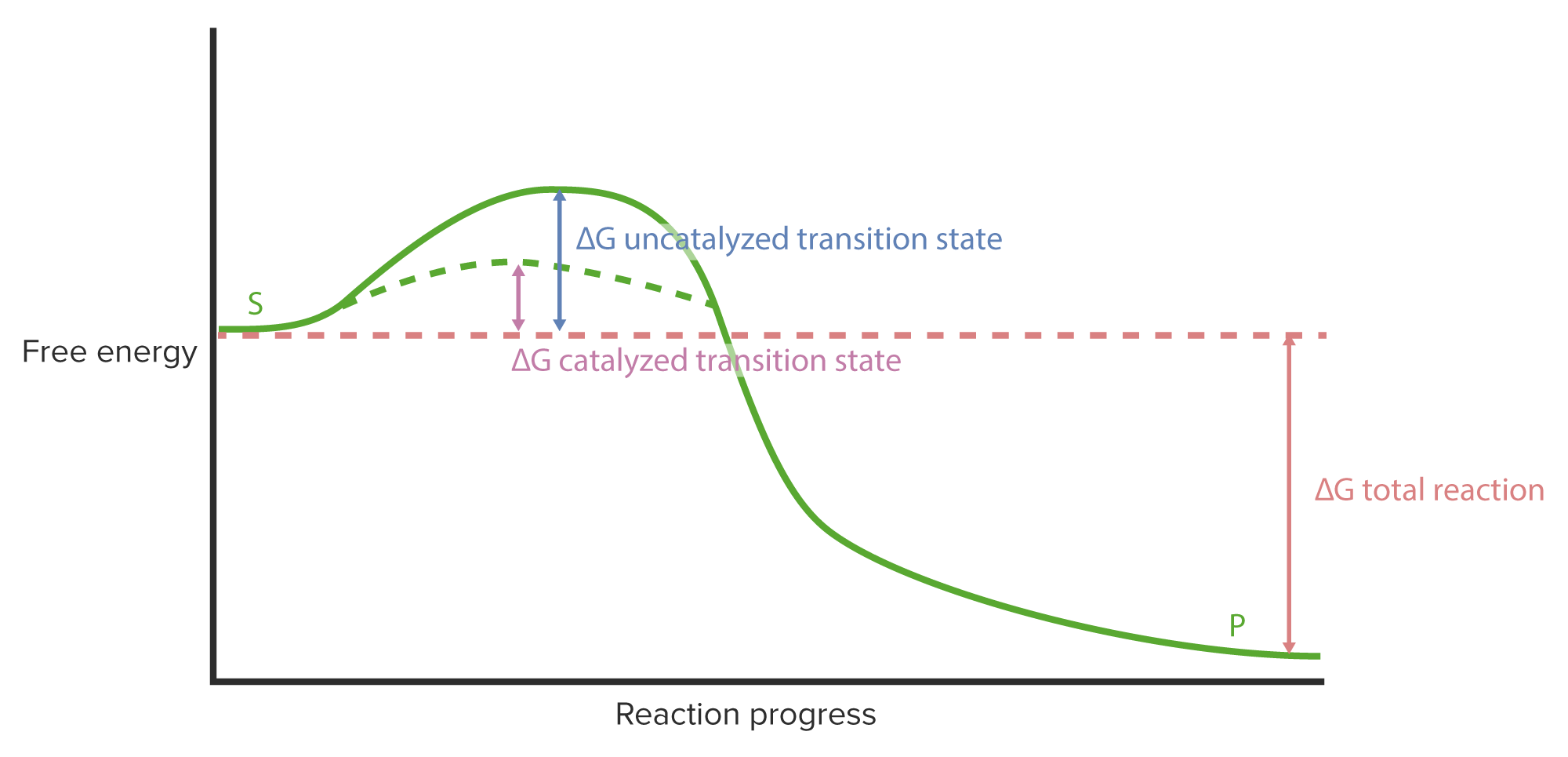
00:01 Enzyme können, wie ich eingangs erwähnt habe, Reaktionen auf unterschiedliche Weise binden, und ich habe darüber gesprochen, wie ein Substrat zu einem Produkt oder zwei Substrate zu einem Produkt umgewandelt werden. 00:11 Oder der Fall, den ich hier beschreiben werde, wie zwei Substrate zu zwei Produkten werden. 00:14 Wenn wir nun an zwei Substrate denken die an ein Enzym binden, begreifen wir, dass es verschiedene Wege gibt, wie sie binden können. 00:21 Wenn wir zum Beispiel die Reaktion A plus B wird zu C plus D haben, könnten wir uns vorstellen, dass vielleicht A zuerst binden müsste und dann B. Oder vielleicht bindet B zuerst und dann A. 00:33 Wir stellen jedoch fest, dass es für einige Enzyme wirklich egal ist, welches Enzym zuerst bindet. 00:39 Dies wird als zufälliger Bindungsmechanismus bezeichnet, wie hier im ersten Beispiel auf dem Bildschirm. 00:44 Zufällige Bindung bedeutet, dass die Reihenfolge keine Rolle spielt. 00:47 Einige Enzyme binden Substrate zufällig, wie ich hier zeige. Aber viele Enzyme setzen hingegen einen geordneten Bindungsablauf voraus. 00:54 Das bedeutet, dass entweder A zuerst binden oder B zuerst binden muss. 00:59 Dieses Modell oder dieser Mechanismus ist maßgeblich, denn: es ist wahrscheinlich die beste Veranschaulichung für das "Induced Fit"-Modell von Koshland, die ich Ihnen geben kann. 01:11 Denn das geordnete Bindungsverhalten sagt uns, dass, wenn eines dieser Elemente zuerst gebunden werden muss - also vor dem Anderen - dies bedeutet, dass die Bindung des Ersten die Form der Bindungsstelle für das Zweite ändert. 01:25 Weil, wenn das Zweite versucht, zuerst zu binden, hat die Änderung noch nicht stattgefunden und deshalb kann der zweite nicht zuerst binden. Also untermauert das Prinzip der geordneten Bindung das Induced Fit-Modell von Koshland. 01:37 Damit scheint das gesamte Gebiet abgedeckt zu sein, aber tatsächlich gibt es noch ein drittes Modell, das Enzyme verwenden, um Reaktionen zu katalysieren, und dieses ist irgendwie interessant und es hat einen lustigen Namen. 01:46 Wir nannten es den Ping-Pong-Mechanismus und er wird auch als Doppel-Verdrängungsreaktion bezeichnet. 01:51 Aber der Sinn ist derselbe. Der Ping-Pong-Mechanismus bezeichnet ein Enzym, das in zwei kovalent unterschiedlichen Zuständen existiert. 02:02 Das bedeutet, dass das Enzym tatsächlich physisch an etwas bindet und eine Änderung verursacht. Dies sehen wir auf der nächsten Folie. 02:09 Dies veranschaulicht nun eine Reaktion von A plus C zu B plus D, und wir sehen, dass es sich in zwei Reaktionen aufspaltet. 02:21 Nun gut. Bei dieser Reaktion passiert Folgendes: A beginnt mit einem Sauerstoff, und bei der Reaktion von A zu B wird der Sauerstoff durch eine Aminogruppe ersetzt. 02:34 Das geschieht also. Und woher kommt die Aminogruppe? Die Aminogruppe kommt vom Enzym. 02:40 Das Enzym transportiert also die Aminogruppe, es trägt sie zu A. 02:45 Wenn also A mit dem Enzym interagiert, tauscht das Enzym seine Aminogruppe, die es trägt, für den Sauerstoff, der sich auf A befindet, ein. 02:55 Auf der rechten Seite der oberen Gleichung sehen wir also, dass A zu B geworden ist; weil es jetzt eine Aminogruppe hat und das Enzym sich den Sauerstoff geschnappt hat. Es hat keine Aminogruppe mehr. Ich habe es grün eingefärbt, damit Sie das sehen können. 03:08 Im zweiten Teil der Reaktion interagiert C, das eine Aminogruppe hat, mit dem Enzym, welches nun einen Sauerstoff besitzt. 03:18 Und wenn das passiert, tauschen sie die Plätze. 03:22 C wird zu D, wobei D eine Doppelbindung zum Sauerstoff ausbildet, und das Enzym hat sich mit der Aminogruppe verbunden. 03:30 Alles klar? Das Enzym ist also zu seinem ursprünglichen Zustand zurückgekehrt. 03:34 Durch diesen Ping-Pong-Mechanismus geht das Enzym also ständig von Aminogruppe zu Sauerstoff, Aminogruppe zu Sauerstoff, und je nachdem in welchem Zustand es sich befindet, bestimmt das Enzym, an welches Substrat es bindet und mit welchem es tauscht. 03:51 Diese Art der Reaktion, die ich Ihnen gerade beschrieben habe, ist eine weit verbreitete Reaktion, die von Enzymen namens Transaminasen durchgeführt wird. 03:57 Transaminasen sind Enzyme, die genau das tun, was ich beschrieben habe. 04:00 Sie tauschen Sauerstoff gegen Aminogruppen aus und dies ist eine sehr wichtige Reaktion im Stoffwechsel der Aminosäuren; denn Aminosäuren erhalten ihre Aminogruppen in einigen Fällen über die Reaktion, die Sie oben sehen. 04:16 Sie beginnen mit einem doppelt gebundenen Sauerstoff und werden zu einem Amin. 04:19 Ein sehr gutes Beispiel dafür ist das Molekül alpha-Ketoglutarat im Zitronensäurezyklus. 04:25 Alpha-Ketoglutarat kann zu Glutaminsäure werden, wenn sein Sauerstoff gegen eine Aminogruppe ausgetauscht wird. 04:32 Auf diese Weise kann die Zelle eine Aminosäure herstellen, die sie vielleicht braucht; dank dieses Mechanismus. 04:39 Auf der anderen Seite haben wir vielleicht die Situation, in der Glutaminsäure oder sogar eine andere Aminosäure zur Energiegewinnung benötigt wird. 04:47 Menschen, die sich zum Beispiel eine low-carb-Diät machen, nehmen nicht viele Kohlenhydrate zu sich. 04:52 Aber sie verhungern nicht, denn sie essen viel Eiweiß. Proteine liefern Aminosäuren. 04:57 Und Aminosäuren liefern Energie als ein Ergebnis dessen, was ich Ihnen hier zeige. 05:02 Die Reaktion unten links hat eine Aminosäure, deren Aminogruppe durch einen doppelt gebundenen Sauerstoff ersetzt wird. Stellen Sie sich vor, dass die Aminosäure auf der unteren linken Seite in Wahrheit Asparaginsäure ist. 05:15 Asparaginsäure kann durch Vertauschen ihrer Aminogruppe mit Sauerstoff zu Oxalacetat werden. 05:23 Und Oxalacetat kann im Zitronensäurezyklus oxidiert werden. 05:26 Diese Transaminasen-Reaktion ist also wichtig sowohl für die Herstellung von Aminosäuren als auch für die Energiegewinnung aus Aminosäuren.
About the Lecture
The lecture Binding of Multiple Substrates – Enzyme Classification by Kevin Ahern, PhD is from the course Enzymes and Enzyme Kinetics.
Included Quiz Questions
Which of the following is true for enzymes that bind to multiple substrates?
- Ordered binding requires one substrate to bind first.
- The ping-pong mechanism refers to the enzyme flipping between T and R states.
- Random binding allows the enzyme to rapidly switch between two different states.
- Random binding is best explained by the Induced fit model.
Which of the following enzyme follows the double displacement mechanism during a biochemical reaction?
- Transaminases
- Ligases
- Lyases
- Hydrolases
- Peroxidases
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
The Professor is excellent, every one can understand easily de concepts