Playlist
Show Playlist
Hide Playlist
Background of Enzymatic Reactions – Enzyme Catalysis
-
01 Advanced Enzymes&Kinetics1.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
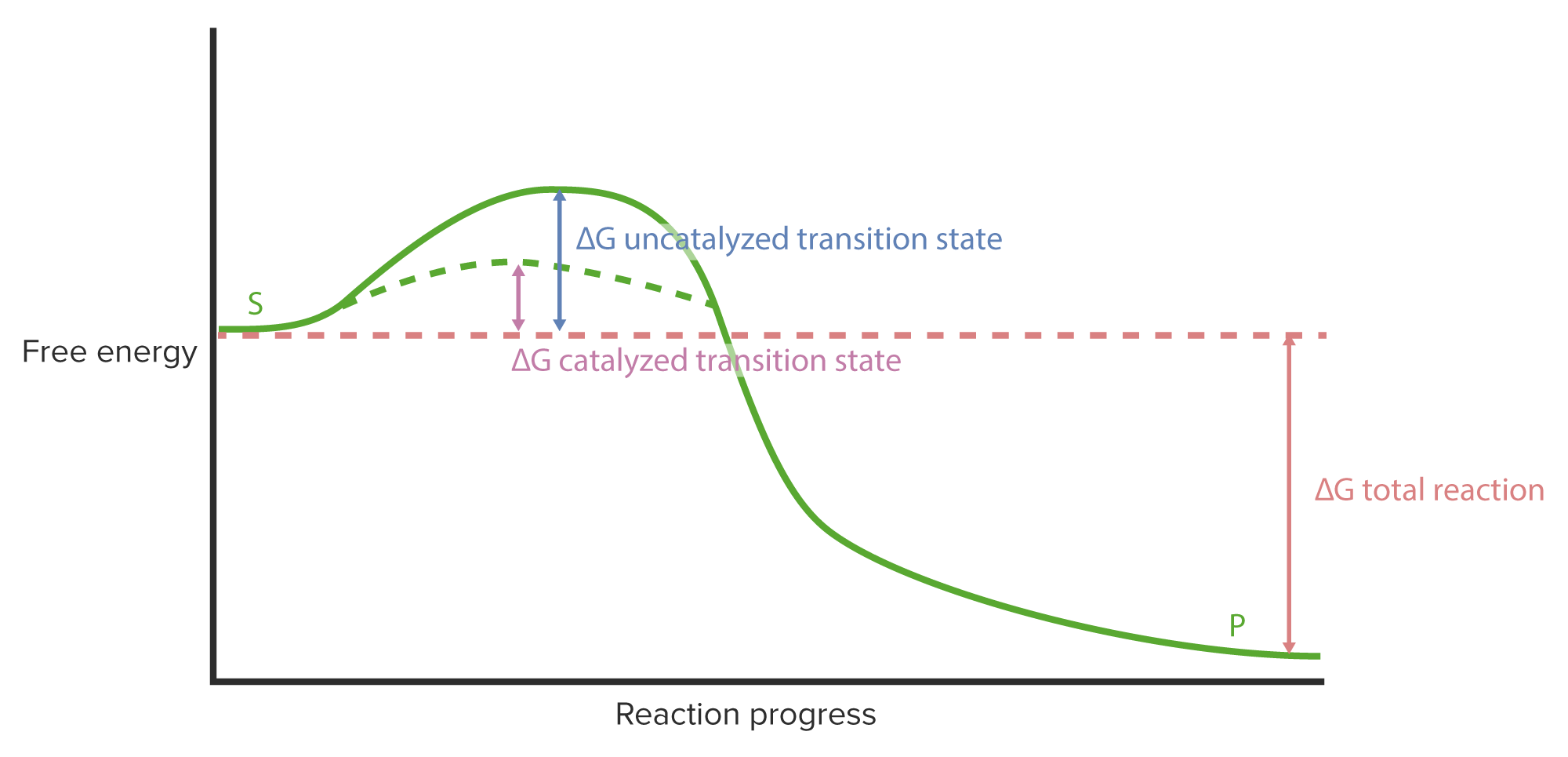
00:02 Die Fähigkeit von Enzymen, Reaktionen zu beschleunigen, ist wirklich verblüffend. 00:06 In dieser Präsentation betrachten wir die Kinetik oder auch die Art und Weise, wie Enzyme Reaktionen beschleunigen. 00:12 In dieser Präsentation werde ich ein paar Hintergrundinfos über den Prozess der Katalyse geben, über die Flexibilität von Enzymen sprechen und darüber, wie dies zu ihrer einzigartigen Funktion beiträgt. 00:21 Ich werde über Aktivierungsenergie - ein weiterer Aspekt der enzymatische Katalyse - sprechen. 00:26 Ich werde über den Mechanismus einer ganz bestimmten Reaktion eines Enzyms namens Serinprotease sprechen. 00:30 Und dann werde ich die kinetischen Überlegungen darlegen, denen wir während dieses analytischen Prozesses begegnen. 00:34 Und schließlich werde ich einen Gesamtüberblick über die sogenannte Michaelis-Menten-Kinetik geben. 00:41 Nun, wenn wir enzymatische Reaktionen betrachten, gibt es eigentlich eine Reihe von verschiedenen Möglichkeiten, wie Moleküle mit einem Enzym interagieren können. 00:49 Es gibt zum Beispiel Reaktionen, die aus einem einzigen Substrat ein einzelnes Produkt ergeben: A wird in B umgewandelt. 00:56 Wir können eine Reaktion durchführen, bei der ein einziges Substrat in mehrere Produkte umgewandelt wird. 01:00 Wenn ich zum Beispiel A nehme und es in zwei Moleküle aufspalte, würde dies B und C ergeben. 01:06 Ich könnte mehrere Substrate nehmen und ein einzelnes Produkt herstellen, was das Gegenteil bedeuten würde, nämlich dass ich zwei Dinge zusammenfüge, um ein drittes zu erhalten; dies wäre dann C, wie hier dargestellt. 01:15 Und schließlich könnte ich mehrere Substrate in mehrere Produkte umwandeln: A und B reagieren zu zwei verschiedenen Dingen, C und D. 01:24 Nun, Enzyme, wie ich schon sagte, können auf wundersame Weise Reaktionen katalysieren und sie sind so viel schneller als ein chemischer Katalysator, dass wir uns vor Augen führen sollten, auf welche Weise sie dies erreichen können. 01:38 Diese Abbildung einer enzymatischen Reaktion veranschaulicht Schritt für Schritt einige der Aspekte, wie Enzyme funktionieren. 01:46 Chemische Katalysatoren - bitte erinnern Sie sich - sind sehr starr. 01:50 Ein Platinkatalysator zum Beispiel atmet nicht. Er bewegt sich nicht. Er ist einfach eine Oberfläche, auf der etwas geschehen kann. 01:58 Und Enzyme unterscheiden sich grundlegend davon. 02:01 Auf dieser Abbildung sehen wir ein Enzym, grün dargestellt, und wir sehen das aktive Zentrum des Enzyms, d.h. der Ort, an dem die Reaktion katalysiert wird, in hellgrüner Farbe. 02:11 Das Enzym in dieser Reaktion, die ich Ihnen zeige, ist eine Reaktion mit mehreren Substraten und mehreren Produkten. Wir werden also A und B haben, wie Sie hier sehen können, und sie werden in zwei andere Moleküle umgewandelt. 02:22 Wir beginnen mit dem nicht-beladenen Enzym: Keine Produkte am Enzym und natürlich auch keine Substrate. 02:29 Substrate sind Moleküle, die an das Enzym binden, und sie werden so gebunden, dass sie sich an dem Ort positionieren, wo die Reaktion stattfindet: am aktiven Zentrum. 02:41 Wir können hier sehen, dass die Substrate begonnen haben, an das Enzym zu binden. 02:43 Das Enzym ist wieder grün eingefärbt. Substrat A hat an den oberen Teil des Enzyms angedockt und Substrat B am unteren Teil. 02:52 Die Wechselwirkung der Substrate mit dem Enzym wird tatsächlich bewirken, dass sich das Enzym zu verändern beginnt. 02:58 Dies ist Koshlands Enzymmodell des "Induced Fit" ("induzierte Passform"). 03:01 Koshlands "Induced Fit" besagt, dass das Enzym nicht nur die Substrate in Produkte umwandelt, sondern, dass - vorübergehend während des katalytischen Prozesses - auch die Substrate das Enzym verändern. Und wie wir sehen werden, ist das für diese Reaktion unerlässlich. 03:19 Die Substratbindung ist also erfolgt. 03:21 An diesem Punkt haben wir den sogenannten ES-Komplex, den Enzym-Substrat-Komplex, gebildet. 03:28 Im nächsten Schritt sehen Sie hier, was passiert ist, nämlich dass die Reaktion abläuft. 03:32 Und das Enzym hat seine Form im Vergleich zur ursprünglichen Bindung leicht verändert, um A und B näher zusammenzubringen. 03:42 Für eine chemische Reaktion ist natürlich Nähe eine absolut notwendige Voraussetzung, damit die Reaktion stattfinden kann. Die leichte Formänderung des Enzyms hat also A und B dazu gebracht, näher aneinander zu rücken. 03:56 Diese Änderungen der Konformation können für ein Enzym sehr groß oder auch sehr subtil sein, aber nichtsdestotrotz findet die Veränderung bei jeder Reaktion statt. 04:06 Jetzt läuft die Reaktion wieder ab, wie wir sehen können, denn die Substrate wurden in in unmittelbare Nähe gebracht. 04:11 Zu diesem Zeitpunkt, während die Reaktion abläuft, liegt der sogenannte ES*-Komplex vor. 04:18 Wir können uns das einfach als den Ort vorstellen, an dem die Reaktion nun stattfinden kann. 04:23 Sehen wir uns dies genauer an: Während der Reaktion sich ein Teil von A nach B bewegt. 04:29 Dies war eine Übertragung eines Teils eines Substrats auf ein anderes. 04:33 A ist nun nicht mehr A und B ist nicht mehr B. 04:38 Wir haben einen Komplex geschaffen, den wir EP-Komplex nennen. 04:42 Wir haben die Produkte hergestellt, aber wir haben die Produkte noch nicht freigegeben. 04:44 A ist also zu C geworden und B ist zu D geworden. 04:49 Jetzt sind die Produkte immer noch vom Enzym umfasst. 04:53 Aber die Produkte sind anders als A und B. 04:56 Genau wie A und B die Form des Enzyms verändern, bewirken auch C und D eine Formveränderung des Enzyms. 05:05 Sie merken wahrscheinlich, worauf das hinausläuft. 05:08 Das Enzym nimmt wieder seine Ausgangsform an. 05:10 Und genau das passiert hier. 05:12 In dieser Reaktion sehen wir, dass sich das Enzym verändert und wieder den Ausgangszustand eingenommen hat. 05:18 Im Ausgangszustand können wir es uns so vorstellen, dass seine Finger ausgestreckt sind - wie meine Hand - und C und D bereit sind, wegzufliegen. 05:26 Das Enzym - nun wieder in seinem ursprünglichen Zustand - ist in der Lage, erneut Substrat zu binden. 05:32 Es ist bereit für den nächsten Prozess. Wenn wir uns nun an die Definition eines Katalysators erinnern, die jeder im Chemieunterricht lernt, ist ein Katalysator ein Molekül oder ein Objekt, das eine Reaktion katalysiert, aber dabei unverändert bleibt. 05:45 Das ist ein Prinzip, das jedem Chemiestudenten im ersten Semester eingebläut wird. 05:50 Jetzt sehen wir, dass Enzyme tatsächlich ein bisschen gegen dieses Prinzip verstoßen. 05:55 Sie werden während des Prozesses vorübergehend verändert, aber zum Schluss sehen sie genauso aus wie am Anfang. 06:00 Insgesamt verstoßen sie nicht gegen das Prinzip, aber sie schummeln ein bisschen. 06:03 Auf dieser Folie sehen wir eine Zusammenfassung aller Reaktionen und Schritte des Prozesses, den Sie bereits kennengelernt haben. 06:08 Ich möchte das nicht noch einmal durchgehen, aber ich möchte Sie darauf hinweisen, dass die Pfeile in beide Richtungen gehen. 06:15 Und das bedeutet, dass diese Reaktion und jeder Schritt dieser Reaktion reversibel ist. 06:20 Nun, Reversibilität einer Reaktion ist eine sehr wichtige Eigenschaft, die Sie sich im Hinterkopf behalten sollten, wenn wir über Stoffwechselprozesse sprechen. Im Übrigen auch bei nicht-metabolischen Prozessen, aber besonders bei metabolischen Prozessen; denn wir müssen uns fragen: "Unter welchen Bedingungen läuft etwas rückwärts ab?" Wir haben gelernt, wie Enzyme über ihre Flexibilität ihre einzigartige Funktion ausüben.
About the Lecture
The lecture Background of Enzymatic Reactions – Enzyme Catalysis by Kevin Ahern, PhD is from the course Enzymes and Enzyme Kinetics.
Included Quiz Questions
During an enzymatic reaction, the substrate binds to which of the following?
- The active site of an enzyme
- The allosteric site of an enzyme
- The site near the hydrophobic regions
- The site near the hydrophilic regions
- The location rich in sulfur-containing amino acids
Which of the following statement is FALSE regarding enzymes?
- During an enzymatic reaction, the enzyme's shape gets changed permanently.
- During an enzymatic reaction, the enzyme brings changes in the substrate molecule or molecules to form one or more products.
- An enzyme speeds up a biochemical reaction by lowering the activation energy.
- At the end of the enzymatic reaction, the enzyme regains its original shape by releasing the product/products from its surface.
- According to the induced fit model of enzyme action, the binding of substrate(s) also causes transient changes in the shape of the enzyme.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
4 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Me encantó como lo ha explicado, paso a paso, de manera detallada y concisa. He aprendido mucho gracias a este video, ¡gracias!
I love it... 100% worth it... Made it simple and easy to understand!
Simplified the lecture in enzymes and i like how everything was explained in a simple but direct way. :)
l am a pre-med student and after watching this lecture I decided I was definitely subscribing! Everything was explained clearly and I understood the concept completely.