Playlist
Show Playlist
Hide Playlist
Argininosuccinate Synthetase
-
Slides UreaCycle Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
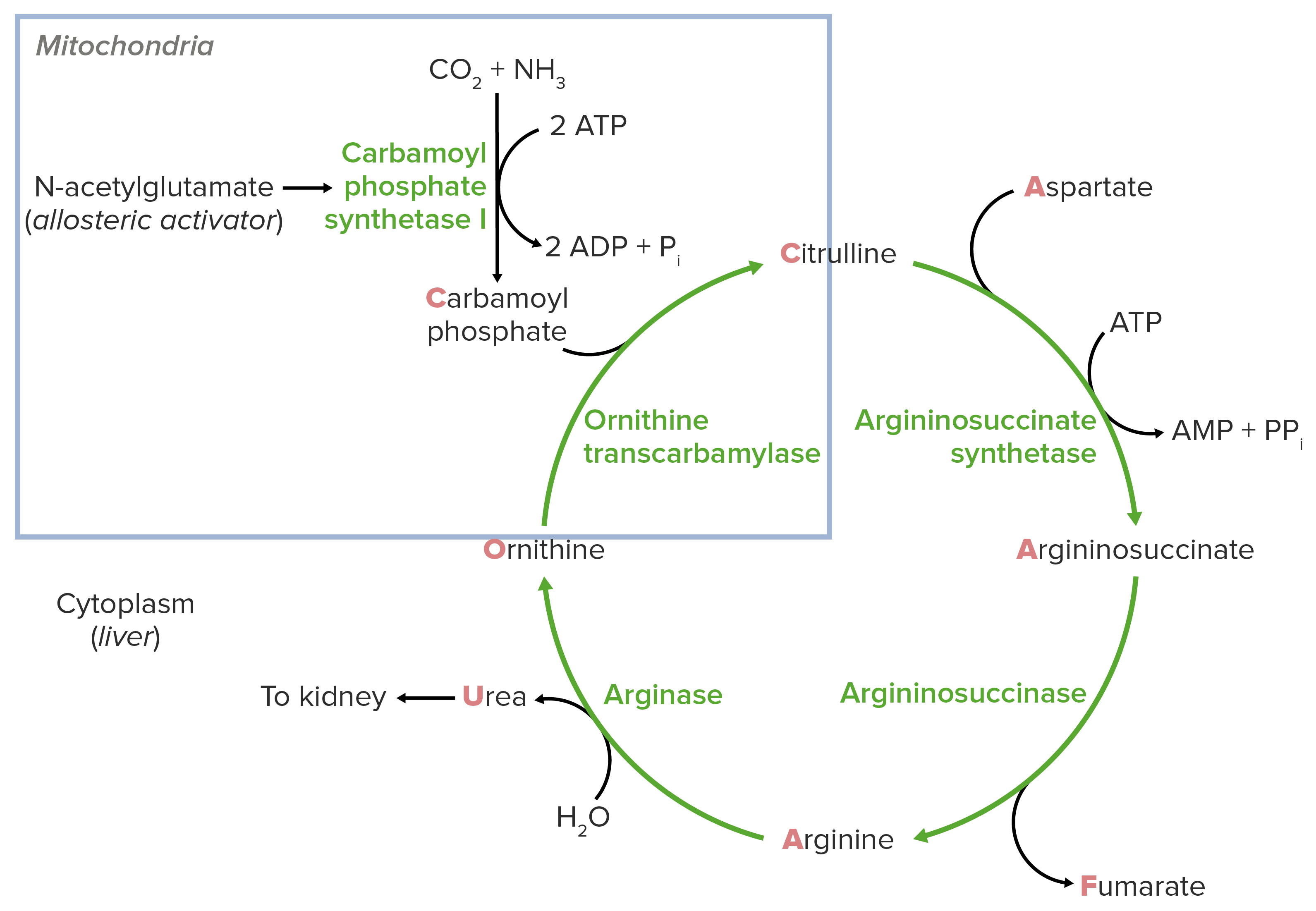
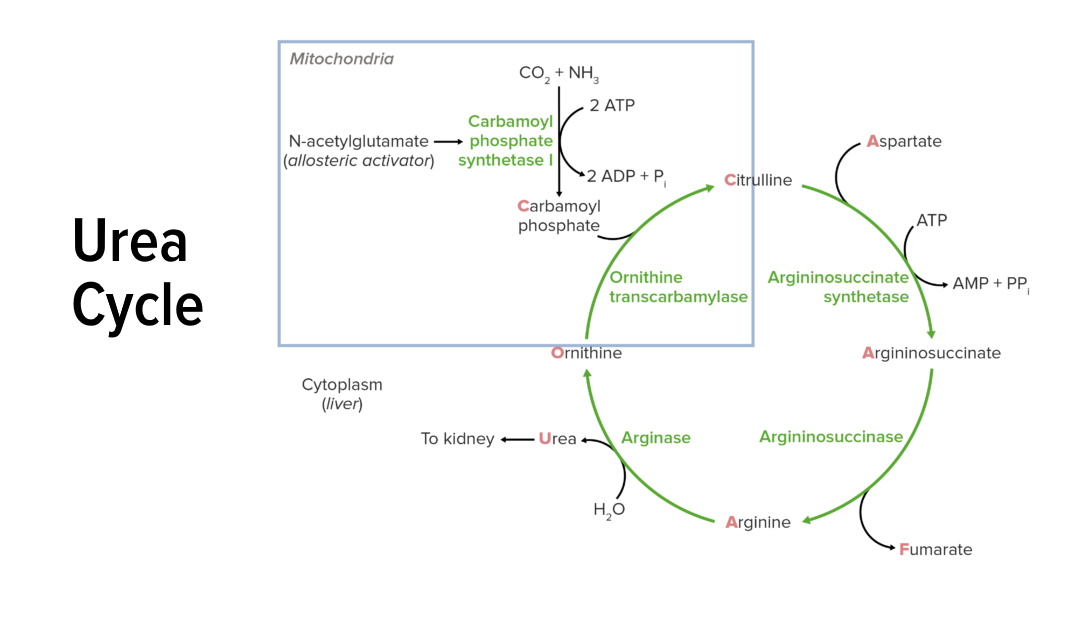
00:02 The next step in the reaction involves the enzyme argininosuccinate synthetase. 00:07 And in this reaction, you remember there's a two-step process. 00:10 In the first step of the process, ATP cleaves off a pyrophosphate. 00:15 We see that happening in that very first step in the top. 00:18 We see a little molecule in blue, PPi. 00:20 That's pyrophosphate. 00:22 That is released from the ATP. 00:24 That allows the remaining part of the ATP in the form of AMP to combine with the citrulline to make the L-citrulline adenylate complex. 00:33 In the second part of the process, aspartate displaces the AMP and that’s where we see the AMP leading also shown in blue to create the L-argininosuccinate. 00:44 The chemical mechanism of the reaction is shown below. 00:47 Now this two-step process, as I said, involves AMP attachment. 00:51 And we see the AMP attachment going here, followed by displacement of the AMP by the aspartate. 00:57 And the displacement is shown here. 01:00 The product to the reaction argininosuccinate is the substrate for the next step of the reaction. 01:05 This reaction is the rate limiting step of the cycle. 01:09 Meaning that it is the slowest and it's the step that other reactions await for it to be completed. 01:16 The gene expression of the enzyme is reduced by arginine. 01:20 Now arginine will see seized further ahead in the pathway. 01:23 And as arginine accumulates, there's no reason to continue making this enzyme. 01:27 So this regulation by arginine, of this enzyme that feeds and makes arginine is important. 01:34 On the other hand, the expression of the enzyme is increased by citrulline. 01:38 And citrulline is further behind that in the cycle and it's literally pushing the thing forward. 01:44 So this kind of regulation ensures that the cell has the proper amount of the argininosuccinate synthetase enzyme. 01:51 Defects in this enzyme lead to citrullinemia which involves an accumulation of ammonia. 01:56 And in fact, most of the enzymes in the urea cycle when their deficient result in accumulation of ammonia and all the consequences that arise as a result of that. 02:06 Like other enzyme deficiencies at the urea cycle, it is treated with a low protein diet and in some cases, with supplementation of arginine which is needed in the next reaction. 02:16 The next step in the process is catalyzed by the enzyme, argininosuccinate lyase. 02:20 We see the structure of the enzyme in the far left of the slide. 02:23 In this reaction, argininosuccinic acid or argininosuccinate, as we describe it, is converted into arginine and fumaric acid. 02:32 This involves a cleaving of the bond that shown as on the screen. 02:38 In this reaction, arginine is produced. 02:40 Now arginine really has two or even three ultimate fates. 02:44 Most commonly, it can go to proteins or it can remain and continue in the urea cycle. 02:49 The fumaric acid or fumarate, as we also call it, can be released and oxidized in the citric acid cycle. 02:56 Now, remember what's happen as we added an aspartic acid in the previous reaction and we've lost fumarate in this reaction. 03:03 The net result was an additional amine got built into our molecule in this case in the form of arginine. 03:09 Now this reaction is very important for the production of arginine. 03:13 Cells need a lot of arginine, not just for the urea cycle but also for making proteins. 03:19 It's also a source of fumarate. 03:21 And the citric acid cycle is a reaction cycle where a lot of energy is produced. 03:25 So having more fumarate available allows the cell to have more energy. 03:29 A deficiency of this enzyme, like that of other urea cycle enzymes, results in excess ammonia accumulation. 03:37 In the next step of the reaction, arginine is cleaved to make urea and ornithine in a reaction catalyzed by arginase. 03:46 Now the ornithine can go ahead and go back to the mitochondria and complete the urea cycle. 03:51 The urea cycle can be excreted into the urine. 03:54 This reaction occurs by cutting the bond shown in arginine here to make the urea. 04:01 Now this enzyme is co-expressed with nitrogen oxide synthase in smooth muscle. 04:06 These two compete for the use of arginine. 04:10 The more arginase available, the more arginase that's used in the urea cycle, the less arginine is available to make nitric oxide. 04:17 So again, we have to balance how much of each of these is use for the cell to properly function. 04:22 Nitric oxide functions and signaling to relax smooth muscle and facilitates the erection of the penis. 04:29 A deficiency of arginase is the rarest of the urea cycle enzymes. 04:33 And the reason for that is because there were two forms of arginase that are present in cells. 04:37 They provide some backup when one is deficient.
About the Lecture
The lecture Argininosuccinate Synthetase by Kevin Ahern, PhD is from the course Amino Acid Metabolism.
Included Quiz Questions
Which of the following is true regarding argininosuccinate synthetase?
- Expression of this enzyme is reduced by arginine.
- It uses a mechanism with a covalent bond to ADP.
- It requires glutamic acid.
- Its deficiency leads to tyrosinemia.
- It catalyzes the fastest part of the reaction.
Which of the following is true regarding argininosuccinate lyase? Select all that apply.
- It releases fumarate.
- Glutamic acid is needed to cleave arginine.
- It is needed for the production of tyrosine.
- When deficient it leads to ammonia excess.
- It produces arginine.
Which of the following is true regarding arginase?
- It competes with nitric oxide synthase for arginine.
- It is the most commonly deficient enzyme of the cycle.
- It produces citrulline and urea from arginine.
- It produces ornithine and citrulline from arginine.
- There is only one form of arginase in cells.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |