Playlist
Show Playlist
Hide Playlist
Thymidylate Synthase and Recycling of Folates
-
Slides NucleotideMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
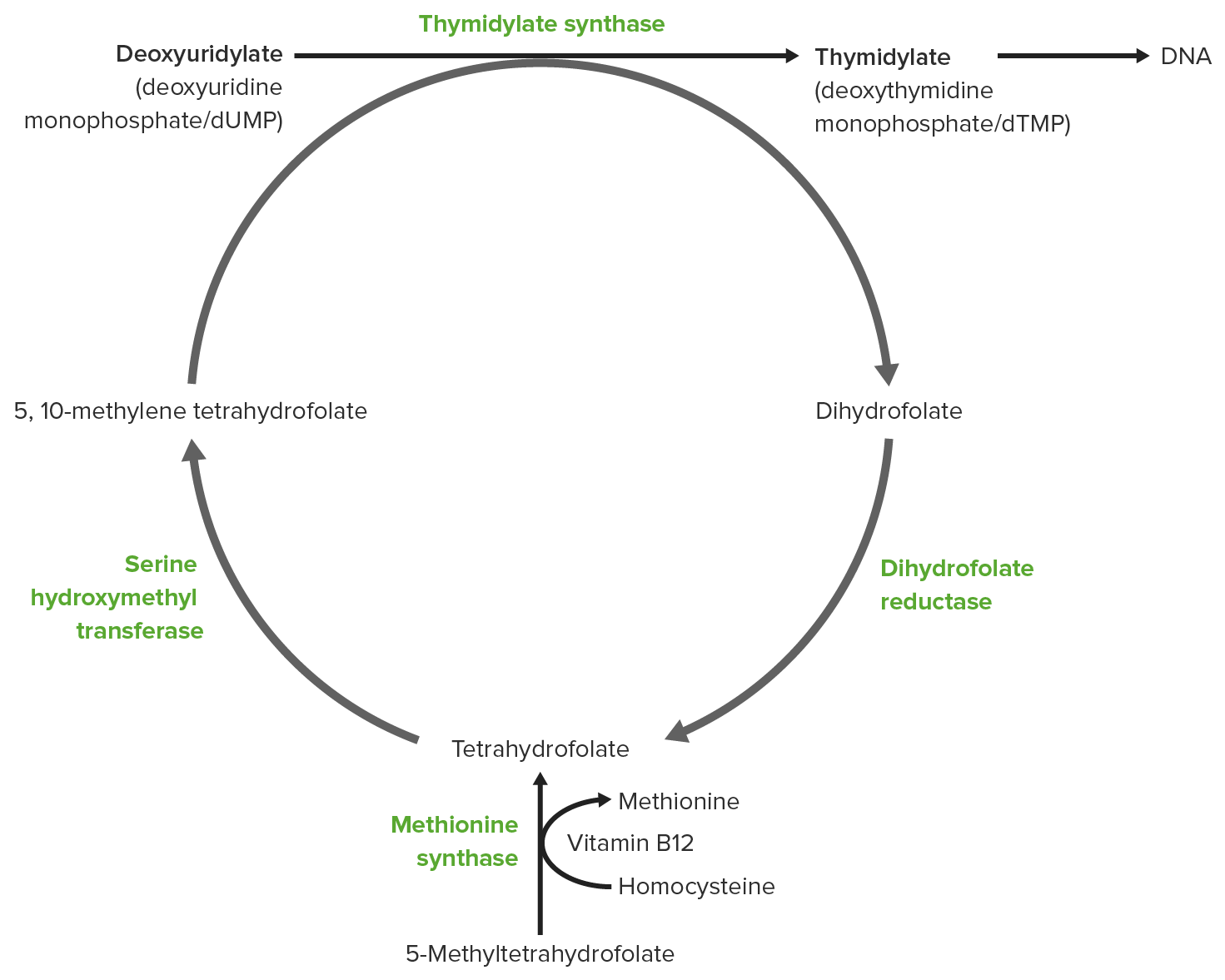
00:00 Schauen wir uns nun die von der Thymidylat-Synthase katalysierte Reaktion zur Umwandlung von dUMP zu dTMP an. Bei dieser Reaktion wird eine zusätzliche Methylgruppe an der Stelle des dUMP verwendet, um dTMP zu machen. Das ist der einzige Unterschied zwischen diesen beiden Molekülen. Diese Reaktion erfordert eine Methylgruppe, die von 5,10-Methylentetrahydrofolat stammt. Wir sprachen darüber, als ich in einem anderen Vortrag über den Folatstoffwechsel sprach. Das Produkt der Spende dieses Kohlenstoffs kommt aus dem 5,10-Methylentetrahydrofolat, das bewirkt, dass dieses Molekül zu Dihydrofolat wird. Die Struktur des Moleküls auf der linken Seite, dessen Namen ich nicht noch einmal nennen werde, ist unten dargestellt und das Dihydrofolat ist dort abgebildet. Der Unterschied zwischen diesen beiden wird mit einem grünen Kasten gezeigt. Der Verlust des Kohlenstoffs ist hier deutlich zu sehen. Also, dieses große Molekül wird verwendet, um diesen einzelnen Kohlenstoff zu spenden. Das ist eine ziemlich wichtige Reaktion, denn ohne sie kann die Zelle kein dTMP herstellen. Ich möchte also ein paar Worte darüber verlieren, wie wir dies Enzym für medizinische Zwecke kontrollieren können. Oben auf dem Bildschirm sehen Sie 5-Fluorouracil. 5-Fluorouracil ist eine Behandlung, die zur Behandlung von Krebszellen eingesetzt wird. Krebszellen, die sich schnell teilen, müssen in der Lage sein reichlich Thymidin-Nukleotide zu bilden. Und das ist die einzige Möglichkeit für die Zelle, Thymidin-Nukleotide herzustellen. 01:25 Wenn man also dieses Enzym hemmt, hemmt man die Fähigkeit der Krebszelle zur Produktion von den Nukleotiden, die eine Zellteilung ermöglichen. 5-Fluorouracil ist also das, was wir als das kennen, was wir als Selbstmordinhibitor bezeichnet. Ein Selbstmordinhibitor ist ein Molekül, das wie ein normales Substrat für das Enzym aussieht. Und das Enzym schnappt sich den Hemmstoff. Aber nachdem es den Hemmstoff geschnappt hat, geht der Inhibitor eine kovalente Bindung mit dem aktiven Zentrum des Enzyms ein und das Enzym begeht dadurch Selbstmord, ohne es zu merken, indem es das Falsche geschnappt hat. Als Folge wird das Enzym außer Kraft gesetzt und die Produktion von Thymidin-Nukleotiden wird gestoppt. Der Krebs kann mit einem Molekül wie diesem gestoppt werden. Wie ich schon sagte, wird es zur Krebsbekämpfung eingesetzt. Eine andere Überlegung bei der Synthese von Thymidin-Nukleotiden involviert die Spende eines einzelnen Kohlenstoffs. 5,10-Methylentetrahydrofolat auf der linken Seite spendet den Kohlenstoff und wird zu Dihydrofolat auf der rechten Seite. Der Verlust dieses Kohlenstoffs, wie ich vorhin gezeigt habe, ist genau dort. Das Problem bei dieser Reaktion, das die Zelle betrifft, ist, dass Dihydrofolat eine Sackgasse in der Reaktion aller Folate ist. Es muss in eines der anderen Folate umgewandelt werden, damit die Folate für andere Reaktionen zur Verfügung stehen. Wenn Dihydrofolat nicht in die anderen Folate umgewandelt wird, dann landen alle Folate in den Dihydrofolat Form und werden nicht verfügbar sein, um Kohlenstoffe für die Herstellung von Thymidin-Nukleotiden geben zu können. 03:09 Diese Umwandlung von Dihydrofolat in Tetrahydrofolat ist daher eine sehr wichtige Reaktion. Wir haben das beim Recycling von Folaten in der Folatvorlesung gesehen. Dihydrofolat-Reduktase katalysiert diese Reduktion, wie Sie sehen können, und das daraus resultierende Tetrahydrofolat kann dann wieder in den Folat-Pool und andere Folate herstellen, die die Zelle benötigt. Diese Dihydrofolatreduktase ist also ein wichtiges Enzym. Die Serin-Hydroxymethyltransferase ist das Enzym, das die Umwandlung der Tetrahydrofolate zurück in die anderen Folatformen katalysiert unter Verwendung eines Kohlenstoffs aus Serin um Glycin herzustellen. Das geschieht hier, und Sie können sehen, dass der Kohlenstoff hier wieder hinzugefügt wird. Also, jetzt hat sich der Kreis geschlossen. Wir haben ursprünglich mit dem 5,10-Methylentetrahydrofolat begonnen. 03:58 Die Thymidylat-Synthase produziert das Dihydrofolat, die Dihydrofolat-Reduktase produziert ein Tetrahydrofolat und die Serin-Hydroxymethyltransferase reproduziert das 5,10-Methylentetrahydrofolat. 04:11 Interessant ist das letzte Enzym, die Serin-Hydroxymethyltransferase. 04:16 Das Plasmodium-Enzym unterscheidet sich von dem menschlichen Enzym. Plasmodium ist an der Entstehung von Malaria beteiligt. Das Plasmodium-Enzym ist also ein gutes Ziel für Studien zur Malariabekämpfung und kann Teil einer wirksamen Behandlung gegen Malaria sein. Nun, auf der anderen Seite, und ich habe dies auch in der Folatvorlesung erwähnt, wenn wir die Dihydrofolatreduktase hemmen können, hemmen wir indirekt die Thymidylatsynthetase-Reaktion. Erinnern Sie sich, daß 5-Fluorouracil ein direkter Inhibitor durch Selbstmordhemmung ist und die indirekte Hemmung dieses Enzyms kommt durch die Behandlung der Zellen durch Methotrexat. 04:55 Dadurch wird die Dihydrofolatreduktase gehemmt. Wenn das geschieht, dann kann die Zelle natürlich nicht diesen Recyclingprozess durchlaufen, den ich gerade beschrieben habe. Daher wird es kein 5,10-Methylentetrahydrofolat für die Thymidylat-Synthase geben und die Thymidylat-Synthese wird stoppen. Die Wiederverwertung von Folaten wird, wie gesagt, gehemmt, und wir können sehen, warum dieses Methotrexat sehr gut bei der Hemmung wirkt. Es sieht dem Dihydrofolat furchtbar ähnlich. Es bindet also an die aktiven Stelle der Dihydrofolatreduktase und konkurriert mit Dihydrofolat um die Wirkung von dem Enzym. Methotrexat wird in der Chemotherapie eingesetzt, und zwar aus demselben Grund, aus dem wir 5-Fluorouracil verwenden. 05:41 Wenn wir die Thymidylat-Synthese hemmen können, dann können wir die Fähigkeit einer Tumorzelle, sich zum Wachstum zu teilen, hemmen. Diese Art der Behandlung ist wirksam gegen sich schnell teilende Zellen, denn erinnern Sie sich, für jede dieser Behandlungen, die wir einem Individuum verabreichen, können sie in auch in eine normale Zelle gelangen und einige der gleichen Auswirkungen auf eine normale Zelle haben. Wenn wir also eine sich schnell teikende Zelle haben und diese Behandlung durchführen, dann ist es viel wahrscheinlicher, dass wir die sich schnell teilende Zelle abtöten als die sich langsamer teilende normale Zelle.
About the Lecture
The lecture Thymidylate Synthase and Recycling of Folates by Kevin Ahern, PhD is from the course Purine and Pyrimidine Metabolism. It contains the following chapters:
- Thymidylate Synthase
- Recycling of Folates
Included Quiz Questions
Which of the following is true regarding thymidylate synthase?
- It transfers a carbon from a folate to make dTMP.
- It catalyzes the removal of a methyl group from dUMP to form dTMP.
- It requires 5-fluorouracil to function.
- All of the answers are true.
- None of the answers are true.
Which of the following is true regarding dihydrofolate reductase?
- It is necessary for folate recycling.
- It is inhibited by dihydrofolate.
- It is inhibited by tetrahydrofolate.
- All of the answers are true.
- None of the answers are true.
Which of the following is true regarding methotrexate?
- All of the answers are true.
- It is an inhibitor of dihydrofolate reductase.
- It is used in chemotherapy.
- It resembles dihydrofolate.
Which of the following is not true regarding suicide inhibitor?
- The suicide inhibitor causes the activation of cancer-causing enzymes and leads to the formation of malignant tissues.
- The suicide inhibitor binds to the enzyme by covalent bonding and causes irreversible enzyme inhibition.
- The suicide inhibitor is usually a substrate analog.
- 5-fluorouracil is a suicide inhibitor of the thymidylate synthase enzyme.
- Due to its suicide inhibitor nature, 5-fluorouracil is used in the treatment of cancerous tissues.
Which of the following descriptions is correct?
- 5,10-Methylenetetrahydrofolate - donates a methyl group to convert dUMP to dTMP
- Methotrexate - stimulates cell division by participating in regeneration of 5,10-methylenetetrahydrofolate
- Thymidylate synthase - converts dTMP to dUMP
- 5-fluorouracil - promotes the conversion of dUMP to dTMP
- Serine hydroxymethyltransferase - promotes the conversion of dihydrofolate into tetrahydrofolate
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |