Playlist
Show Playlist
Hide Playlist
Polysaccharides
-
Slides 04 MacromoleculesI CellBiology.pdf
-
Reference List Molecular and Cell Biology.pdf
-
Download Lecture Overview
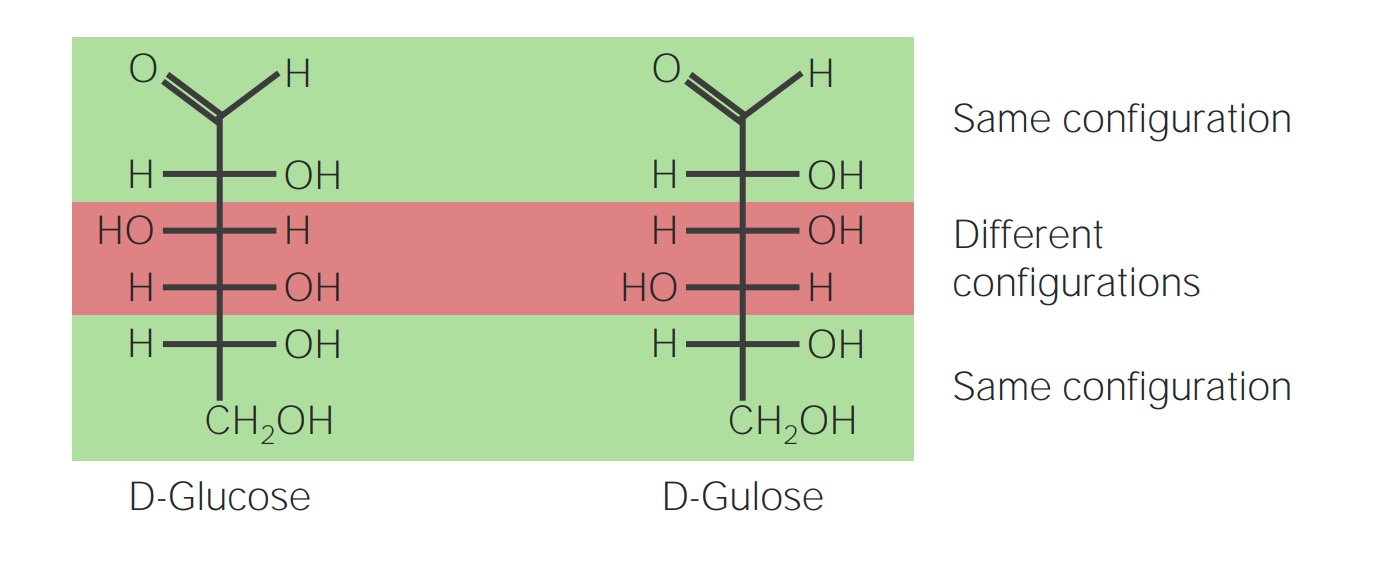
00:00 So, polysaccharides are more than two sugar monomers so more than two monomers of sugar strung together could form starch or glycogen or chitin for that matter. 00:13 Here's an example of glycogen, a storage polysaccharide that we see in our muscle cells. 00:21 This is how we store glucose, comes into the blood, we pack it away and stored as glycogen in the muscles or in the liver. 00:28 We could also see amylose or amylopectin; this is a storage polysaccharide that we see in plants. 00:35 Cellulose is also a linear chain of glucose molecules strung together. 00:42 So, the question is then, why is it that we couldn't eat grass like a cow could? Again, it comes down to enzymes specificity and this isomerization issue. 00:53 This is an isomer molecule where we see not the alpha glycosidic linkage or the alpha form of the molecule, we see beta glucose where the OH and H group are reversed and this is a beta 1, 4 glycosidic linkage and we don't contain any enzymes ever in our life to break down this polysaccharide so cows actually have bacteria in their gut that help them break down these bonds, they have the bacteria, have the enzymes that break down the beta glycosidic linkage so that cows can actually then breakdown the carbon chains and release energy and make ATP and live off of that ATP. 01:36 An example of a structural polysaccharide would be chitin. 01:41 Chitins found in the shells of lobsters and crabs and shrimp and it's made of a chain of glucose molecules that are cross linked with proteins to give it much more integrity and strength. 01:52 Again, we couldn't eat the shell of a lobster or crab or you could try but it wouldn't be so good. 01:59 So the take home message here about the group of polysaccharides is that the monomer is called the monosaccharide, the polymer is a polysaccharide no matter which storage form or structural form it is and the linkage between them is by dehydration synthesis but it's called a glycosidic linkage because it is in carbohydrates so carbohydrates have a monomer, a polymer and a linkage. 02:31 And for each of the macromolecules were going to discuss the monomer, the polymer, and the linkage form between them. 02:39 So hopefully from this lecture, you have a nice introduction to each of the macromolecules that we're going to cover what their monomers, and what their polymers were called. 02:50 and you could explain the process of dehydration synthesis, how each of the monomers are held together as well as identify some carbohydrate structures like glycogen, and amylopectin and chitin and discuss precisely why you can't eat grass to your friends? Thanks so much for joining me for this lecture I hope to see you in the next one shortly.
About the Lecture
The lecture Polysaccharides by Georgina Cornwall, PhD is from the course The Macromolecules of Life.
Included Quiz Questions
Which of the following is TRUE about polysaccharides?
- They are composed of monosaccharides linked by glycosidic bonds.
- Cellulose monomers are joined by alpha linkages.
- They are only used as energy-storage molecules by organisms.
- They are present only in animals.
- They can be metabolized by any enzyme in the digestive system.
Why can't cellulose be metabolized by humans?
- Because humans lack the enzyme that breaks the β-glycosidic bond between glucose molecules
- Because it is composed of α-glucose molecules
- Because it is crosslinked with proteins
- Because bacteria in the human digestive system inhibit the breakdown
- Because the monomers of cellulose are made of ribose
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Easy to understand and fun! Really useful! Great examples are provided!