Playlist
Show Playlist
Hide Playlist
Osmosis and Tonicity – Transport Across Cell Membranes
-
Slides 08 TransportAcrossCellMembranes CellBiology.pdf
-
Reference List Molecular and Cell Biology.pdf
-
Download Lecture Overview
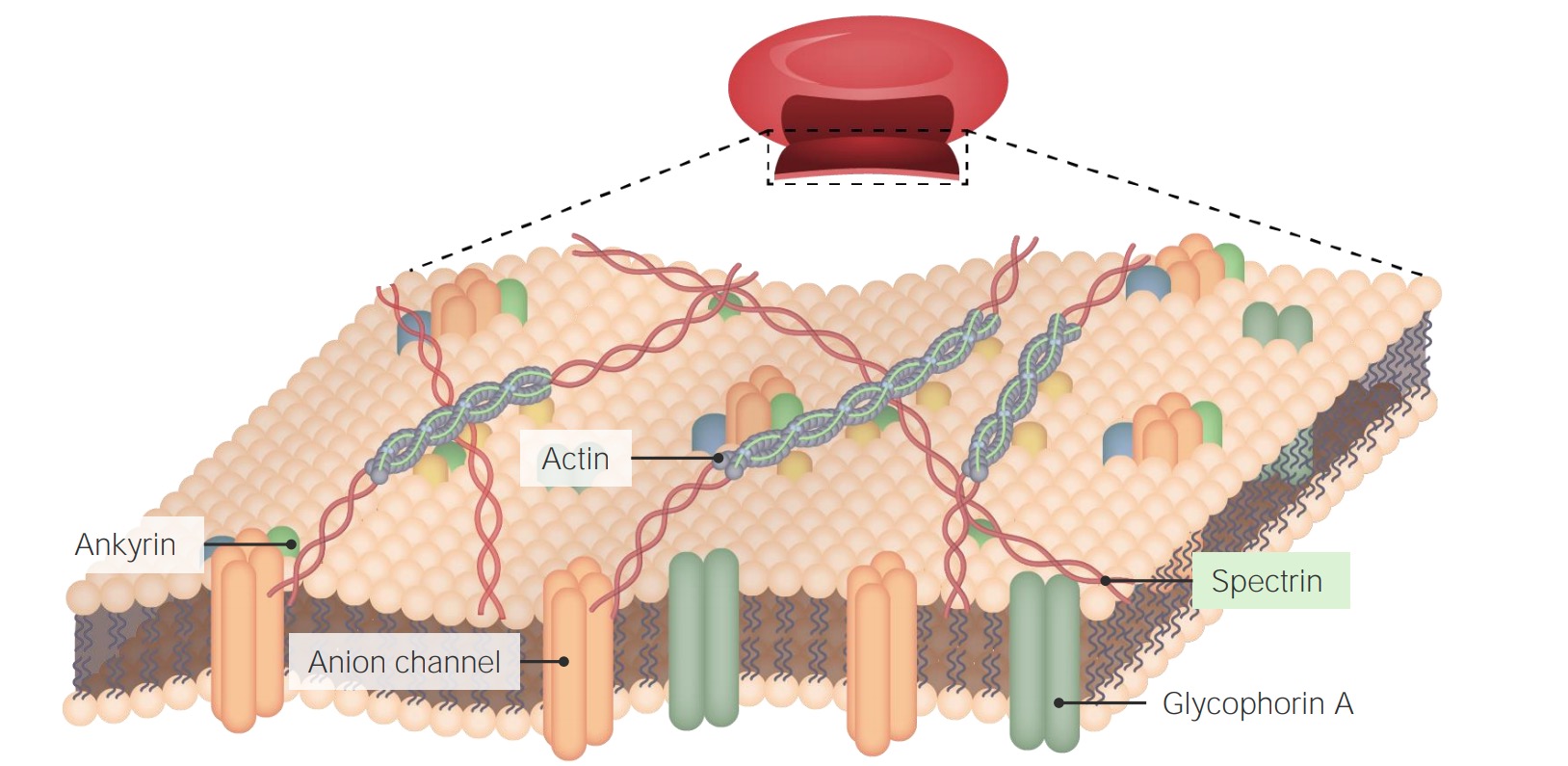
00:00 Dann könnten wir eine Situation haben, in der die Proteine nicht in die Membran eingebettet sind. Und Sie haben eine hohe Konzentration von Stoffen auf einer Seite der Membran und eine niedrige Konzentration von Stoffen auf der anderen Seite der Membran. Was passiert in dieser Situation wenn die Moleküle die Membran nicht passieren können, weil es keine Kanalproteine oder Trägerproteine gibt?. Sie wollen aber in die Zelle hinein. Alles im Leben will ins Gleichgewicht kommen. 00:31 Hier ist also ein U-Rohr. In der Mitte befindet sich eine Membran. In diesem Rohr haben wir eine Menge gelöster Stoffe auf einer Seite der Membran. Und dann haben wir wenige gelöste Stoffe auf der anderen Seite der Membran. 00:46 Die gelösten Stoffe befinden sich in einem Lösungsmittel. Das Lösungsmittel ist hier einfach die wässrige Umgebung, wie wir sie auch in einer Zelle sehen würden. Das Wasser kann also durch eine Zellmembran hindurch gelangen, aber sollte es das auch? Warten Sie einen Moment. Warum sollte Wasser die Zellmembran durchdringen können? Ist Wasser nicht ein polares Molekül? Haben wir nicht diesen riesigen hydrophoben Bereich durch die hydrophoben Schwänzen der Phospholipide? Das Wasser ist so klein, dass es sich tatsächlich irgendwie dazwischen schleichen kann. Es gibt einige andere Mechanismen, die wir lernen werden, wie das Wasser trotzdem durchkommt. Aquaporine und dergleichen. Wir werden uns aber jetzt noch nicht näher damit befassen. 01:31 So oder so, nehmen wir der Einfachheit halber an, dass Wasser so klein ist, dass es sich einfach direkt zwischen den Phospholipiden hindurchmogelt. Nicht komplett wahr, aber möglich. Was passiert also um alles ins Gleichgewicht zu bringen? Das Wasser fließt durch die Membran vom Ort der hohen Konzentration der gelösten Stoffe zum Ort der niedrigen Konzentration der gelösten Stoffe. 01:55 Das wird klar, wenn wir uns die Bewegung innerhalb des U-Rohrs ansehen, in dem sich das Wasser von einer Seite zurück zur anderen Seite bewegt, um eine gleichmäßig konzentrierte Lösung auf beiden Seiten der Membran zu schaffen. Übertragen wir das auf eine zelluläre Umgebung, um es auf die Realität zu übertragen. Die Bewegung des Wassers wird durch den osmotischen Druck bestimmt. Der osmotische Druck treibt das Wasser in den Bereich der höheren Konzentration, um die Lösung auf dieser Seite der Membran zu verdünnen. 02:40 Tonizität (effektive Osmolarität) heißt das Konzept und dazu schauen wir uns als Beispiel rote Blutkörperchen an. 02:47 Nehmen wir an wir haben eine biologische Umgebung so wie sie sein sollte. Unsere Blutzellen sind die roten Blutkörperchen. Sie schwimmen in unserem Blut herum. Die Lösung im Inneren der roten Blutkörperchen sollte die gleiche Tonizität oder Osmolarität haben wie die Lösung, in der die roten Blutkörperchen selbst schwimmen. 03:04 Auch das ist eine Frage der Homöostase. Wir können sagen, dass diese Lösungen isotonisch oder isosmotisch sind. Je nachdem welche Terminologie Sie bevorzugen. Normale Zellen bewegen sich also in normaler extrazellulärer Flüssigkeit, die die gleiche Tonizität hat. Es handelt sich um isotonische Lösungen. 03:28 Was passiert aber, wenn wir unsere roten Blutkörperchen in eine hypertone Lösung geben? Es ist sehr wichtig, welche Lösung hyperton ist. 03:44 Ist es innerhalb der Zelle hyperton oder ist es hyperton außerhalb der Zelle. Als Hinweis: Wir haben Zellen, die eine normale Osmolarität aufweisen, wie sie sie auch in unseren menschlichen Körper haben würden. Und wir geben sie in ein Glas voll mit hypertonem Wasser. 04:05 Als würden wir sie ins Meer kippen. Dort sind ebenfalls viele, viele Stoffe gelöst. 04:09 Was wird mit der Nettobewegung von Wasser passieren? In welche Richtung wird die Osmose das Wasser in diesem Fall drängen? Da die Lösung außerhalb der Zelle hyperton ist im Vergleich zur Lösung innerhalb der Zelle, wird das Wasser in der Zelle nach außen wandern wollen, um zu versuchen, die äußere Umgebung zu verdünnen. 04:33 Nun, das ist keine gute Situation für die roten Blutkörperchen, weil sie dadurch schrumpfen und ihr gesamtes Wasser verlieren können, denn das Wasser kann nicht die ganze Welt verdünnen. 04:42 Wir betrachten hier also die externe Umgebung. Die externe Umgebung ist hyperton gegenüber der inneren Umgebung der roten Blutkörperchen, die hypoton ist im Vergleich zur äußeren Umgebung. Ich weiß, dies kann ein wenig verwirrend sein, aber versuchen wir es indem wir die Situation umgekehrt betrachten. Wir nehmen wieder unsere roten Blutkörperchen, die in eine normale Lösung gehören, mit normaler Osmolarität, also in eine isotone Lösung, so wie wir sie in unserem Körper vorfinden würden. 05:16 Aber irgendwie haben wir keine gelösten Stoffe mehr für das Blut. Wir nehmen also unsere roten Blutkörperchen und geben sie in eine hypotone Lösung. Also in eine Lösung, die weniger gelöste Stoffe hat als die Lösung im Inneren der roten Blutkörperchen. Was wird mit dem Wasser passieren? Bewegt sich das Wasser in die Zelle hinein oder aus der Zelle heraus? Nun, da die roten Blutkörperchen mehr gelöste Stoffe enthalten als in ihrer Umgebung vorhanden sind, wird Wasser in die Zelle einströmen um die Lösung in der Zelle zu verdünnen und die Konzentration mit der Lösung außerhalb der Zelle auszugleichen. Wir bewegen uns damit in Richtung isotonisch. 06:06 Aber die roten Blutkörperchen haben nur eine begrenzte Größe, und durch den hohen Druck, den die Bewegung des Wassers erzeugt kann es schließlich dazu kommen, dass die roten Blutkörperchen platzen. Es ist infolgedessen wichtig bei der Osmolarität oder Tonizität, dass Sie überlegen, welcher Ort hyperton oder hypoton ist. Reden wir über die Umgebung, die die Zelle umgibt oder sprechen wir über das Innere der Zelle selbst? Allgemeinen reden wir darüber, ob wir die Zellen in eine hypotone oder hypertone Lösung geben. Jetzt kommen wir zu einem kleinen Rückblick. 06:45 Wir haben isotonisch. Das ist der ideale Zustand, wie es im menschlichen Körper sein sollte. 06:51 Wenn die Homöostase aus dem Gleichgewicht gerät, kann es passieren, dass ein hypertones Milieu entsteht. Zu viel Salz ist dann in der Umwelt. Wir haben gesehen, dass zu viel Salz die Zellen schrumpfen lässt, weil das Wasser aus den Zellen tritt um die Umgebung zu verdünnen. 07:11 Und wenn wir dann unsere Blutzellen in eine eine hypotone Lösung geben, versucht das Wasser von außen das Innere der Zelle zu verdünnen, indem es hinein strömt. 07:24 Das kann die Zelle zum Platzen bringen oder zum Lysieren, was auseinanderbrechen heißt.
About the Lecture
The lecture Osmosis and Tonicity – Transport Across Cell Membranes by Georgina Cornwall, PhD is from the course Cellular Structure.
Included Quiz Questions
What would happen to red blood cells if the plasma becomes hypertonic, secondary to extreme dehydration?
- Osmotic pressure would pull water out of the cells and they may shrivel.
- Osmotic pressure would draw water into the cells and they may lyse.
- No changes
- Red blood cells would expend energy to maintain their shape and function.
- The life span of cells would be shortened by half.
Which of the following best describes the mechanism of osmosis?
- Water molecules move across a semipermeable membrane from a region of lower solute concentration to a region of higher solute concentration.
- Amphiphilic molecules move across a semipermeable membrane from a region of normal solute concentration to a region of lower concentration.
- Hydrophobic molecules move across a semipermeable membrane from a region of lower concentration to a region of higher concentration.
- Water molecules move across a semipermeable membrane from a region of lower concentration to a region of higher concentration.
- Hydrophilic molecules move across a permeable membrane from a region of higher solute concentration to a region of lower solute concentration.
What is the driving force of osmosis?
- Osmotic pressure
- Atmospheric pressure
- Absolute pressure
- Vacuum pressure
- Gauge pressure
Customer reviews
3,5 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
1 |
| 1 Star |
|
0 |
The pictures really help to understand these processes. Material about physics is usually boring for me but this time is was not so
not very engaging with the viewer, and it could be even more dynamic