Playlist
Show Playlist
Hide Playlist
Nucleotide Metabolism: Introduction and De novo Purine Metabolism
-
Slides NucleotideMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
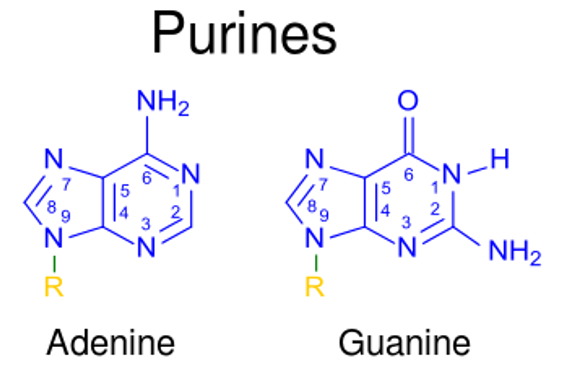
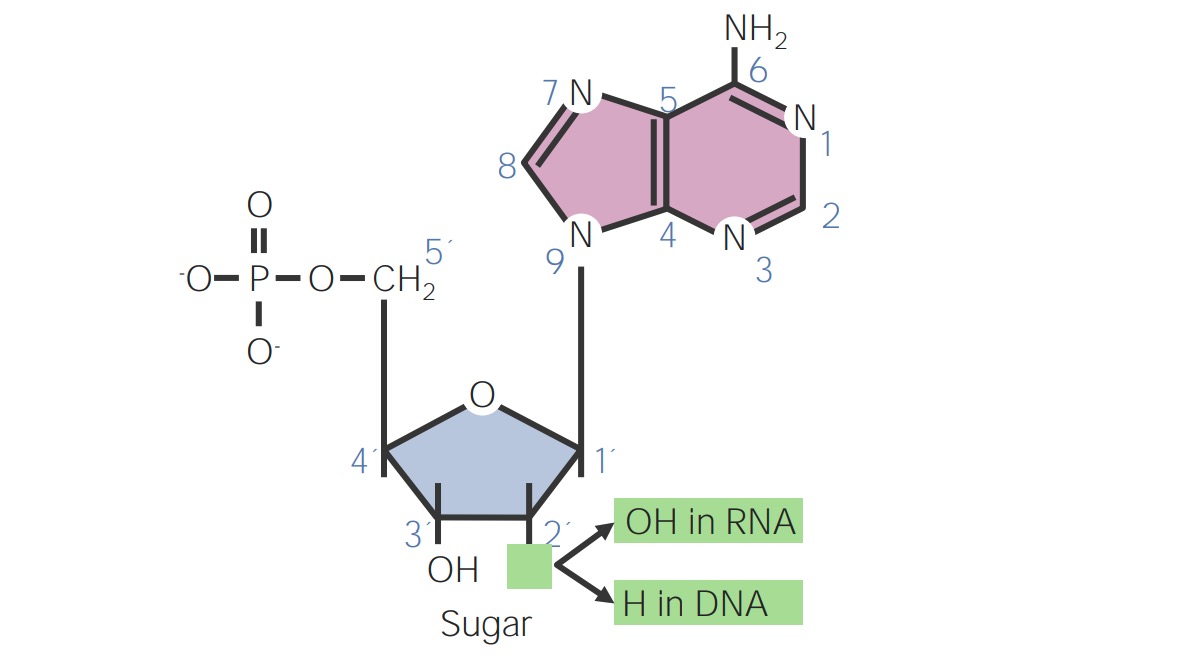
00:00 Praktisch jedes Schulkind lernt heute, dass ATP, GTP, CTP und UTP die Nukleotide sind, die zur Herstellung von RNA verwendet werden, und die Desoxyribonukleotid-Versionen davon sind die Bausteine, die zur Herstellung von DNA verwendet werden. Was sie nicht wissen und was Sie wahrscheinlich nicht gelernt haben ist, wie diese Nukleotide selbst hergestellt werden, und das wird das Thema dieser Vorlesungen sein. Ich habe damit begonnen über die Struktur der Nukleotide zu sprechen, wie wir hier sehen können, und einige Nomenklaturen einzuführen damit wir alle auf dem gleichen Level sind. Alle Nukleotide enthalten 3 verschiedene Komponenten. Die erste davon befindet sich in der Mitte dieser Struktur auf der linken Seite, und das ist die Pentose. Eine Pentose ist eine 5fach-Kohlenstoff Zucker. Auf der rechten Seite ist in blau eine Base zu sehen, die an die Pentose angehängt ist. Diese Base entspricht entweder einem Purin- oder einem Pyrimidinpaar. Die Purine sind Adenin und Guanin, die Pyrimidine Cytosin, Uracil oder Thymin. Der dritte Bestandteil eines Nukleotids ist mindestens ein Phosphatrest, auf der linken Seite in rot dargestellt. Diese Phosphate können einfach, doppelt oder dreifach sein, wie Sie auf dem Bildschirm hier sehen. Die Basen werden im Wesentlichen durch ihre Größe unterschieden. Die Purine, natürlich, mit einem 2-Ring-System, Adenin und Guanin, und die Pyrimidine mit einem einzigen Ring, einer einfacheren Struktur, Cytosin, Uracil und Thymin. Nun sind Nukleotide, wie schon sagt, die Bausteine der DNA und RNA. Die Zellen stellen ihre Nukleotide auf 2 verschiedenen Wegen her. Ein Weg wird als de-novo-Synthese bezeichnet, was bedeutet, dass diese Nukleotide vollständig neu hergestellt werden auf der Basis von sehr, sehr einfachen Verbindungen. Der andere Weg ist die Verwendung von recyceltem Material der Salvage-Pathway, und wie der Name schon sagt, bedeutet das, dass diese Nukleotide hergestellt werden, indem man Stücke von anderen Nukleotiden verwendet, die abgebaut wurden. Purine werden auf einem anderen Weg hergestellt, der sich von dem Weg zur Herstellung von Pyrimidinen unterscheidet. Wir werden über sie also getrennt sprechen. Die Desoxyribonukleotide, aus denen die DNA besteht, werden aus Ribonukleosid-Diphosphaten hergestellt. Um DNTPs herzustellen, müssen wir also zuerst die Ribonukleotid-Versionen davon herstellen. Zuletzt werden Thymidin-Nukleotide aus Uridin-Nukleotiden hergestellt, wie wir sehen werden. 02:26 Um also Thymin für den Einbau in die DNA herzustellen, müssen wir zuerst das Uridin-Äquivalent herstellen. Und nun, wie schon erwähnt, Nukleotide werden aus sehr einfachen Komponenten hergestellt: Aminosäuren, 1 Kohlenstoffspender, und Kohlenstoffdioxid. Wir können das in der Abbildung hier sehen. Wenn wir uns die Quellen der Atome, die zur Herstellung von Purinen verwendet werden anschauen, sehen wir, dass sie nicht sehr kompliziert sind. Wir sehen in grün eine Reihe von Atomen, die von der Aminosäure Glycin stammen. In lila sehen wir die Atome, die von Glutamin stammen. Wir sehen auch ein einzelnes Kohlenstoffatom aus Kohlenstoffdioxid, ein Stickstoffatom aus Asparaginsäure und 2 weitere Kohlenstoffatome, die von Folasaeurederivaten stammen. Diese wurden in einem anderen Vortrag dieser Reihe besprochen. Wenn wir uns die Pyrimidine ansehen, ist es noch einfacher. Nur 3 Komponenten sind notwendig, um den Ring des Pyrimidins zu bilden: Kohlenstoffdioxid, Glutamin und Asparaginsäure. 03:24 Und diese 3 Komponenten wurden auch zur Herstellung von Purinen verwendet. Wir denken also, dass die Herstellung von Nukleotiden, nicht schwer zu verstehen ist, da sehr einfache Vorstufen benötigt werden. Die Synthese der Nukleotide wird sehr stark reguliert. Das ist ein sehr wichtiges Konzept, das man verstehen muss, weil die Zelle das richtige Verhältnis von Purinen zu Pyrimidinen braucht, und jede der einzelnen Zellen vergleicht deren Nukleotide zueinander. Der Grund dafür ist, dass die Zelle, die in irgendeiner Weise aus dem Gleichgewicht ist und sich viele Nukleotide herausnimmt, viel wahrscheinlicher an einer Mutation leidet. Mutationen sind normalerweise schlecht fuer die Zellen und Zellen gehen zu außergewöhnlichen Verbindungen ueber, um so oft wie moeglich zu vermeiden, dass dies so oft passiert. Die Purinsynthese unterscheidet sich von der Pyrimidinsynthese dadurch, dass das Purin die Synthese mit der Anlagerung des Rings an den Ribosezucker beginnt. Die Pyrimidin-Synthese synthetisiert zuerst den Ring und bindet ihn dann später an den Zucker. Während wir den Nukleotid-Stoffwechsel studieren, können wir ihn auf verschiedene Art und Weise betrachten. Wir können ihn aus einem größeren Bild heraus betrachten, wie ich es getan habe bisher, oder wir können heranzoomen und uns auf einzelne Reaktionen konzentrieren. Nun, wenn ich meistens über den Nukleotid-Stoffwechsel spreche, verwende ich gerne die verkuerzte Version, denn während die einzelnen Reaktionen wichtig sind, die wirklich wichtige Botschaft ist nicht, wie genau die einzelnen Prozesse ablaufen, sondern vielmehr, wie der Gesamtprozess abläuft und wie das von mir beschriebene Gleichgewicht aufrechterhalten wird beim Zusammenbau der Purin- und Pyrimidin-Nukleotide. Betrachten wir das Ganze nun in einem größeren Zusammenhang, dann sehen wir, dass das Ausgangsmolekül ein Molekül namens Ribosebiphosphat ist. Das ist die Quelle des Ribose-Pentose-Zuckers, der dort vorkommt. Auf der linken Seite können Sie sehen, dass es mehrere Schritte gibt, um vom Ribosebiphosphat zu einem Zwischenprodukt zu gelangen, und ich werde diese kurz durchgehen, bekannt als IMP, Inosinmonophosphat. IMP ist ein Weichenpunkt. Sie können sehen, wie diese Weichen vom IMP abwärts gestellt sind. Eine Seite geht nach links und bildet letztendlich das unterste ATP und die andere Seite, die nach rechts geht, bildet GTP. Dies sind die 2 Purinnukleotide, die wir herstellen. Und jetzt, das Wichtigste ist, wenn wir zum Punkt des IMP gelangen, dass dies einer der Orte ist, wo das Gleichgewicht hergestellt wird, um die richtigen Mengen von jedem zu erzeugen. Wir werden auch sehen, dass es vor diesem Punkt ein Gleichgewicht gibt, das ganz am Anfang steht und das vielleicht ein wenig schwierig zu verstehen ist, aber ich denke, wenn wir das Gesamtbild betrachten, wird es Sinn machen. Also zuallererst wird bei Ribosebiphosphat ein Pyrophosphat an den Kohlenstoff Nummer 1 angehängt und wir sehen, dass das in diesem Prozess passiert, und wenn ich mir die rechte Seite unten ansehe, sehen wir, dass 2 Phosphate hinzugefügt worden sind. Diese 2 Phosphate stammen vom ATP und das Produkt dieser Reaktion ist AMP. Das Enzym, das diesen Prozess katalysiert, ist als PRPP-Synthetase bekannt. 06:29 Die PRPP-Synthetase ist wichtig, damit die Zelle entscheiden kann, ob sie diesen Prozess startet oder nicht. 06:36 Es handelt sich also um ein regulierendes Enzym, und wir werden gleich sehen, wie diese Regulierung erfolgt. In der nächsten Reaktion beginnen wir mit der Synthese des Purinrings oberhalb der Ribose. Wir sehen also, dass das Diphosphat auf der rechten Seite geändert wurde und durch eine Aminogruppe ersetzt wurde, wie Sie hier sehen, um Phosphoribosylamin herzustellen. Wie ist das Amin dorthin gekommen? Nun, das Amin wurde als Ergebnis der Transaminierungsreaktion hergestellt. Als ich über den Aminosäurestoffwechsel in einer anderen Vorlesung gesprochen habe, haben Sie vielleicht festgestellt, dass die Transaminierung ein wichtiger Weg war, um die Amine über eine Art Austausch auf eine Verbindung zu übertragen, und ein üblicher Weg, diesen Austausch durchzuführen, war die Verwendung von Glutamat oder Glutamin-Aminosäuren. Wir können hier sehen, dass Glutamin, die GLN, die Quelle für das Amin ist und, das Glutamin, das sein Amin verloren hat, macht Glutamat, das ist das GLU, und das Amin bleibt dann am Phosphoribosylamin. Das Enzym, das diese Reaktion katalysiert, ist bekannt als PRPP- Aminotransferase, und auch das ist ein sehr wichtiges Enzym, das der Zelle hilft, zu kontrollieren, welche Nukleotide hergestellt werden, und auch das werden wir gleich sehen. Der Aufbau eines Purinrings umfasst insgesamt etwa 7 oder 8 Schritte. Es handelt sich um eine ziemlich komplizierte Reihe von Reaktionen, und meine Idee ist wenn ich Ihnen diese Reaktionen zeige, nicht Sie dazu zu bringen, die einzelnen Reaktionen auswendig zu lernen, sondern um Ihnen den Prozess zu zeigen, mit dem der Ring beginnt, sich zusammenzusetzen. 08:13 Das Produkt der letzten Reaktion war Phosphoribosylamin und wir sehen, dass Glycin dazu gekommen ist. Wo wir vorher ein Amin hatten, sehen wir jetzt, dass ein Glycin daran angehängt wurde und wir sehen, wie der Ring auf der rechten Seite langsam Form annimmt. Dort ist der Ribosezucker, dort ist der Phosphatrest, und das ist die Basis, die jetzt gebildet wird. Der nächste Schritt des Prozesses fügt mehr an dieses Glycin an, das da war, und wir sehen es wachsen, und jetzt haben wir gesehen, wie wir begonnen haben, einen der beiden Ringe zu bilden oder wie wir ihn vollständig gebildet haben. Der zweite Ring wächst und wächst und wächst und wächst. Jetzt haben wir den zweiten Ring fast fertig. 08:58 Unten links kann man sehen, dass der erste Ring, der gemacht wurde, der untere ist und der zweite Ring sich ihm fast angeschlossen hat. Mit dem nächsten Schritt wird dieser Ring geschlossen.
About the Lecture
The lecture Nucleotide Metabolism: Introduction and De novo Purine Metabolism by Kevin Ahern, PhD is from the course Purine and Pyrimidine Metabolism. It contains the following chapters:
- Nucleotide Metabolism - Introduction
- De novo Purine Metabolism
- Building a Purine Ring
Included Quiz Questions
Which of the following are true regarding nucleotides? Select all that apply
- They are made by de novo and salvage pathways.
- They are made in separate, non-overlapping pathways for each nucleotide.
- They can have any of three purines or two pyrimidines.
- Structurally, pyrimidines have two rings, and purines have one.
- They are the building blocks of DNA and RNA.
Which of the following is true regarding purine synthesis?
- All of the answers are true.
- PRPP synthetase catalyzes the process that converts ATP to AMP.
- It begins with ribose-5-phosphate.
- It includes the intermediate known as IMP.
- It consumes ATP.
Which of the following does NOT take part in ATP and GTP synthesis?
- Gluteraldehyde
- Glycine and glutamine
- CO2
- Aspartate
- N10-formyl-THF
Which of the following is NOT correctly associated regarding ATP and GTP synthesis?
- N10-formyl-THF — source of N atoms in purine synthesis
- Ribose-5-phosphate — starting point
- Inosine monophosphate — branch point between ATP and GTP synthesis
- PRPP synthetase — regulatory enzyme for purine synthesis
- PRPP amidotransferase — helpful in synthesis of phosphoribosylamine
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Thanks for focusing on the "big picture!" The lecturer at my school bogged us down with the details, so it was hard to take in what the most important steps were