Playlist
Show Playlist
Hide Playlist
Nucleoside Analogs and Deoxyadenosine Analogs
-
Slides NucleotideMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
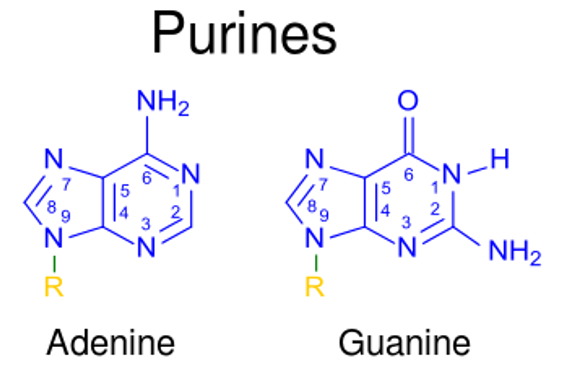
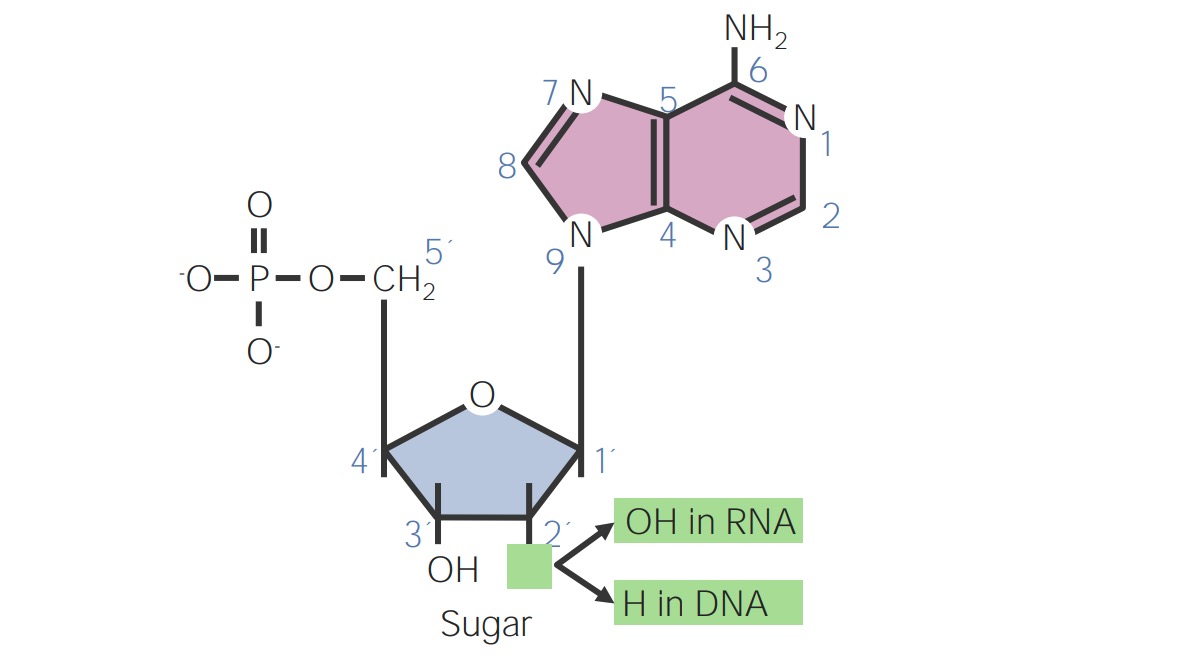
00:00 Nun sind wir alle Überlegungen zur Synthese von Nukleotiden und Nukleoside durchgegangen und Sie haben etwas über die Bedeutung dieser Strukturen gelernt, die in Beziehung zu Enzymen wie DNA-Polymerase und RNA-Polymerase stehen und zur Herstellung von DNA und RNA gebraucht werden. Nutzung dieses Wissens zur Entwicklung von Nukleotidderivaten und zur Hemmung spezifischer Enzyme wie die DNA-Polymerase oder die RNA-Polymerase können wirksam sein, um Infektionserreger, die die menschliche Gesundheit beeinträchtigen, zu stoppen oder zu töten. Diese Nukleosid- und Nukleotidanaloga, die ich jetzt beschreiben werde, sind für diesen Zweck bestimmt. Nun gibt es Nukleosid-Analoga, nicht Nukleotide, die zum größten Teil als Medikamente verabreicht werden, obwohl sie zu Nukleotiden werden, da die Nukleoside die Zellmembran leicht passieren können. Nukleotide können das nicht. 00:55 Der Unterschied zwischen einem Nukleotid und einem Nukleosid ist, dass ein Nukleotid ein Phosphat hat und ein Nukleosid nicht. AMP ist also ein Nukleotid, weil es ein Phosphat hat und Adenosin ist das entsprechende Molekül ohne das Phosphat. 01:12 Adenosin ist ein Nukleosid. Alle Medikamente, die ich Ihnen hier beschreibe, sind also als Nukleoside konzipiert. 01:19 Ihr Ziel ist es, die Zellmembranbarriere zu überwinden. Wenn sie dann in der Zelle sind werden sie von verschiedenen Kinasen phosphoryliert, um mit den nativen Substraten zu konkurrieren: ATP, GTP, UTP, CTP für die Bindung an die Polymerasen. Diese Phosphorylierung ist also entscheidend nachdem es in die Zelle gelangt ist. Nun werden die Analoga, wie gesagt, in der Zelle phosphoryliert und die Bergungssysteme, über die wir vorhin gesprochen haben, sind für diesen Prozess von entscheidender Bedeutung. Lassen Sie uns nun einige dieser einzelnen Nukleotide/ Nukleosid-Analoga betrachten, um zu verstehen, was sie bewirken und wie ihre Struktur mit ihrer Fähigkeit, als antimikrobielles Mittel zu wirken, zusammenhängt. Deoxydenosin hat eine Struktur, wie sie auf dem Bildschirm zu sehen ist. Es fehlt das Hydroxyl an Position 2, das ihm damit den Namen Desoxy gibt. Es hat ein Adenin, das als Base gebunden ist. Ein Derivat dieser Verbindung an dem Didanosin oder DDI sieht aus wie die Struktur, die Sie links sehen. Es hat 2 Unterschiede zwischen Di- und Desoxyadenosin. Erstens enthält es anstelle von Adenin das modifizierte Base Hypoxanthin. 02:26 Hypoxanthin war eine Form von Adenin, die bei der Reaktion gefunden wurde. Es verhält sich, was die Zelle anbelangt, sehr ähnlich wie ein Adenin. Der zweite Unterschied besteht darin, dass der dreipolige Teil des Riboseringes kein Hydroxyl enthält. Erinnere Dich bitte die Molekularbiologie mit DNA-Polymerase und RNA-Polymerase, das 3-Prim-Hydroxyl ist für das Anhängen des nächsten Nukleotids beim Aufbau eines DNA-Moleküls notwenid. 02:59 Das Hydroxyl an der 5er-Position kann verwendet werden, um das modifizierte Nukleotid mit dem vorherigen Nukleotid zu verbinden. Wenn es aber keine Behandlung durch Hydroxyl gibt, kann das nächste Nukleotid nicht angehängt werden. 03:11 Das bedeutet, dass Didanosin während der DNA-Synthese ein so genannter Kettenabbrecher ist. 03:19 Damit dieses Ding funktioniert, muss es zunächst phosphoryliert werden. Also setzen wir dies als Nukleosid ein. Es wird phosphoryliert und von der DNA-Polymerase verwendet. Und dort ist die DNA-Kette beendet. Diese Verbindung wird hauptsächlich als Anti-HIV-Mittel eingesetzt, weil die HIV-DNA-Polymerase dies als Substrat für den Aufbau von DNA erkennt, aber stecken bleibt, da sie sich nicht mehr als Kette ausdehnen kann, wenn sie erst einmal in das wachsende DNA-Molekül eingebaut ist. Eine weitere verwandte Verbindung ist Vidarabin oder auch als Ara-A bekannt. Ara-A hat ein Hydroxyl an Position 2. Es hat ein Adenin als Basis, ist also dem Desoxyadenosin sehr ähnlich. Allerdings ist das Hydroxyl in einer merkwürdigen Position. Sie können sehen, dass es nach oben zeigt und wenn wir eine Ribo-Version hätten, würde es nach unten zeigen. 04:12 Diese Verbindung hier enthält den Zucker Arabinose anstelle von Desoxyribose. Die Ähnlichkeit ermöglicht es diesem Molekül von einer viralen DNA-Polymerase gebunden zu werden, aber es kann nicht richtig funktionieren und nicht richtig an ein zu synthetisierendes DNA-Molekül angehängt werden. Es ist also ein kompetitiver Inhibitor für dATP und verstopft daher die virale Polymerase, so dass sie nicht mehr funktionieren kann. 04:40 Diese Verbindung wird daher als antivirales Mittel eingesetzt. Das Herpesvirus ist eines der Viren, gegen die es häufig zur Behandlung verwendet wird.
About the Lecture
The lecture Nucleoside Analogs and Deoxyadenosine Analogs by Kevin Ahern, PhD is from the course Purine and Pyrimidine Metabolism.
Included Quiz Questions
Which of the following is true regarding nucleoside analogs used in therapeutics?
- They are commonly competitive inhibitors of nucleotides when present in cells.
- They are commonly partial agonists of nucleotides when present in cells.
- Nucleotides move into cells more easily than these nucleoside analogs.
- These analogs are dephosphorylated once they enter the cell.
- These analogs cannot be phosphorylated because of their structure.
Nucleoside and nucleotide analogs commonly used as anti-viral therapeutic drugs to prevent what?
- Viral replication in the infected cells
- Viral adhesion to the cell surface receptors present on the outer surface of the cell membrane
- Viral replication by halting the movement of ribosomes
- Viral replication by halting the assembly of viral proteins
- Viral replication by facilitating the formation of defected viral protein coats
What is a benefit of nucleoside analogs?
- They readily cross the cell membrane.
- They readily cross the blood-brain barrier.
- They are excreted through the spleen.
- They are filtered through the duodenum.
- They have an unknown half-life.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |