Playlist
Show Playlist
Hide Playlist
Dehydration Synthesis and Hydrolysis Reactions
-
Slides 04 MacromoleculesI CellBiology.pdf
-
Reference List Molecular and Cell Biology.pdf
-
Download Lecture Overview
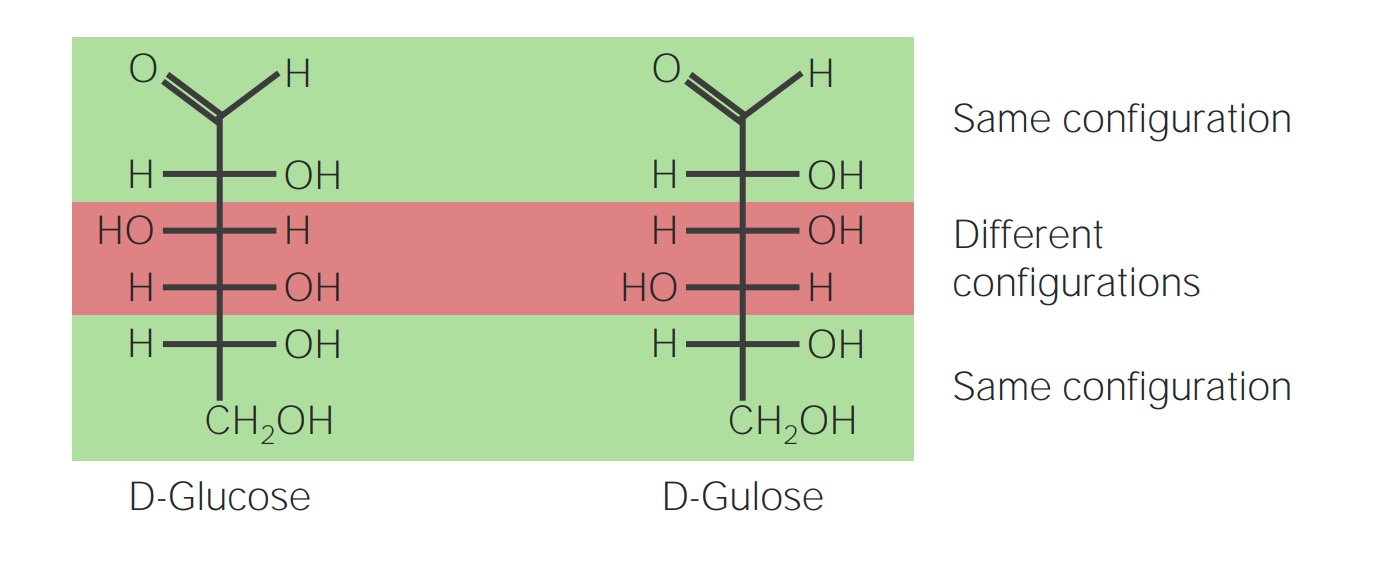
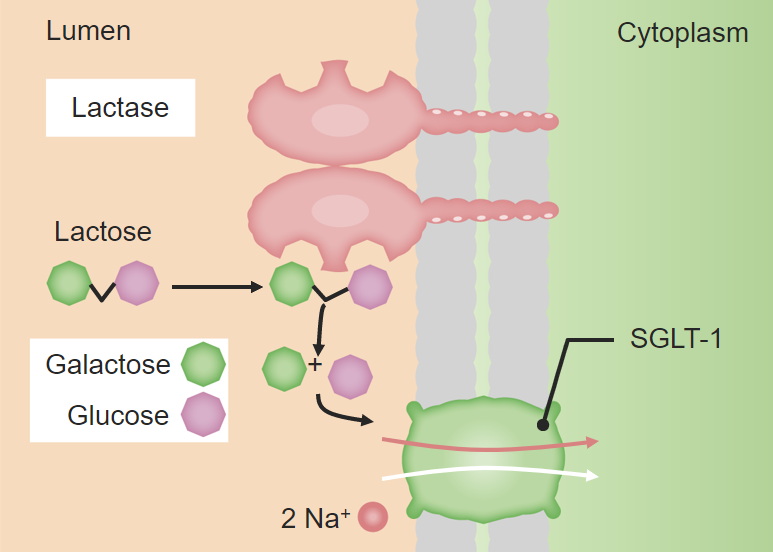
00:01 Then we'll move on into looking at how these come together. 00:04 Macromolecules are all stuck together with our friend the covalent bond. 00:09 What happens here is we remove a hydrogen from one end of the molecule and a hydroxide from the other end of the next molecule, the next monomer and we extract H2O and those two molecules will bind together, they now have an affinity to pair electrons with each other so because we're losing water this is called dehydration synthesis so two monomers will come together through polymerization reaction polymerization where we lose water and that creates the affinity between the two monomers to form a covalent bond and again the covalent bonds are the strongest bonds that we see. 00:57 Single, double and clearly triple the most strong bond we'll see in biology. 01:04 On the other hand, when we metabolize polymer macromolecules for example in the metabolism of say pasta, a complex carbohydrate we're going to separate the monomers from each other in a process called hydrolysis. 01:20 Hydrolysis so we're breaking apart the water molecule. 01:26 We'll put hydrogen on one end of a monomer and hydroxide group on the other end of a monomer and now they're free to separate and that covalent bond is broken between the monomers so contrasting reactions that we see are the hydrolysis reaction breaking things apart and dehydration synthesis is bringing things together.
About the Lecture
The lecture Dehydration Synthesis and Hydrolysis Reactions by Georgina Cornwall, PhD is from the course The Macromolecules of Life.
Included Quiz Questions
What happens when two monosaccharides combine?
- Removal of hydrogen and hydroxide
- Consumption of a water molecule
- Formation of a weak bond
- Formation of a triple covalent bond
- Hydrolysis
What bond is formed during the dehydration of monomers?
- Covalent bonds
- Ionic bonds
- Polar bonds
- Non-polar bonds
- Reactive bonds
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
a very good professor! actually i think that u need to understand this in chemie but is a good thing that she teachs this again. and sorry for my bad english