Playlist
Show Playlist
Hide Playlist
Heme Synthesis
-
Slides HemeSynthesis,IronTransport,Storage.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
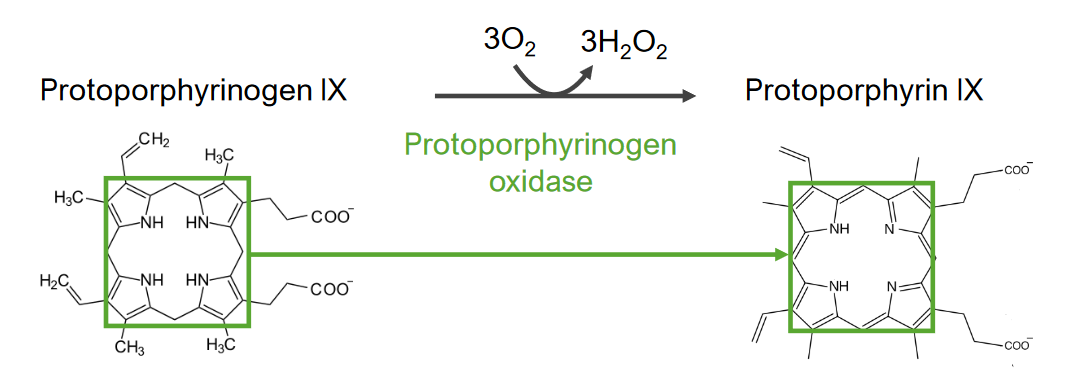
00:02 Now, in going through heme synthesis, what's remarkable is how we can start with very simple molecules and make the complex heme ring as we will see. 00:10 The first step of the synthesis begins with the simple compound, succinyl-CoA and glycine. 00:15 Succinyl-CoA is from the citric acid cycle and glycine is a simple amino acid. 00:22 The combining of these two molecules together creates aminolevulinic acid. 00:27 The first intermediate on the way to making heme. 00:31 This reaction is a decarboxylation that releases both the carbon dioxide and the Co-A part of the molecule as we see here. 00:39 The enzyme catalyzing this reaction is aminolevulinic acid or ALA synthase as we call it. 00:45 ALA synthase is found in almost all non-plant eukaryotes and also in some bacteria. 00:52 Now the enzyme is found in the mitochondria and that's important because that's where the succinyl-CoA is located. 00:58 The synthesis of the enzyme is tightly controlled by the presence of iron binding proteins. 01:03 We don’t want to be making this if iron is not available. 01:06 So, if iron binding proteins are abundant, that means there's iron to put in and make heme. 01:13 The second step in the process of making heme involves the condensation of two of these molecules of aminolevulinic acid. 01:20 The product to this reduction creates porphobilinogen as you can see here. 01:24 And we already start to see complex molecules resulting from these interactions. 01:31 This reaction splits out two molecules of water and it's catalyzed by the enzyme porphobilinogen synthase. 01:39 Porphobilinogen synthase is sensitive to magnesium concentration and also to pH. 01:45 It's also very easily inactivated by heavy metals. 01:48 And because of this, it is actually the source of our sensitivity in our body to lead poisoning. 01:53 When you heard lead poisoning, it's affecting this enzyme. 01:56 And that's pretty severe as you can imagine because if you couldn’t make heme because this enzyme was deficient, you would be in big trouble. 02:04 Four porphobilinogen molecules combine with splitting out of ammonia to create the next molecule hydroxymethylbilane. 02:12 Here are the four porphobilinogens and here's the hydroxymethylbilane. 02:17 Now, I've gone through and marked in the molecules we'll see in a second where these came from. 02:22 The reaction involves the addition of water, the loss of four protons and the loss of four ammonias. 02:29 Again, ammonias need to be dealt with and I've talked about that in another lecture. 02:33 The enzyme catalyzing this is porphobilinogen deaminase. 02:37 And we can see here that the molecules are joined through their amine rings. 02:43 Here, here, here and here. 02:46 So, each box contains one of the precursor porphobilinogens. 02:51 The reduced activity of the enzyme results in intermittent porphyria. 02:55 And intermittent porphyria, as we will see, can cause some really unusual things in terms of behavior in a person. 03:02 In the next reaction, uroporphyrinogen III is made and this is the first cyclic intermediate of the pathway. 03:09 Here's the precursor molecule from the last reaction, hydroxymethylbilane. 03:13 This is a simple reaction involving the loss of water to make uroporphyrinogen III. 03:18 The reaction is catalyzed by the enzyme uroporphyrinogen III synthase. 03:24 In this reaction, we can see what happens. 03:26 The green box on the left shows the place where the reaction occurs. 03:29 And on the right, the water has been lost and a bond has been created to basically fasten together the bottom part of the molecule. 03:36 This now creates a central ring structure in the middle. 03:41 Hydroxymethylbilane can also spontaneously cyclize to form a related compound known as uroporphyrinogen I. 03:48 We won't follow that here. 03:50 A deficiency of this enzyme is associated with another kind of porphyria known as congenital erythropoietic porphyria. 03:57 In the next step of the process, decarboxylation of the uroporphyrinogen III gives us coproporphyrinogen III. 04:04 That is a pretty mouthful of word. 04:07 Uroporphyrinogen III on the left goes to coproporphyrinogen III by the splitting out of these four carbon dioxides. 04:15 We see that happening here. 04:17 Here's where the carbon dioxide comes from. 04:19 And here's what's produced after it has been lost. 04:21 Here's where the second one comes from and the product. 04:24 The precursor and the product, and the precursor and the product. 04:30 The enzyme catalyzing this reaction is uroporphyrinogen III decarboxylase. 04:37 Now, this enzyme is notable because it has an extraordinarily high rate of catalysis compared to the same uncatalyzed reaction. 04:45 In fact, this rate increase of 1024 is the highest of any known enzyme. 04:52 Mutations in this enzyme cause familial porphyria cutanea tarda and hepatoerythropoietic porphyria. 04:59 In the next step of heme synthesis, coproporphyrinogen III is converted to protoporphyrinogen IX through two sequential steps of oxidative decarboxylation. 05:09 And we can see these here. 05:11 Here's the coproporphyrinogen III and here's the protoporphyrinogen IX. 05:16 This reaction involves molecular oxygen, lost of two waters and the two carbon dioxides that I mentioned. 05:23 Coproporphyrinogen III oxidase catalyzes this reaction. 05:27 Here's the precursor on the left and here's the product after the decarboxylation has occurred. 05:33 The precursor on top and the product after the decarboxylation has occurred. 05:38 Reduced amounts of coproporphyrinogen III oxidase leads to hereditary coproporphyria. 05:46 Coproporphyrinogen III oxidase is a mitochondrial, iron-carrying enzyme. 05:51 In the seventh step of this process, protoporphyrin IX is formed from protoporphyrinogen IX by oxidation. 05:58 It's the protoporphyrin IX that is the precursor in which the iron is inserted to make heme. 06:04 Protoporphyrin IX is, I said, the precursor. 06:07 It is the precursor of the hemes, cytochromes and also the hemes that's used in chlorophyll. 06:12 We can see that reaction occurring here. 06:15 The protoporphyrinogen IX on the left and the protoporphyrin IX on the right. 06:19 In this reaction, three molecules of oxygen are added and three molecules of hydrogen peroxide are produced. 06:26 You may recall that hydrogen peroxide is a reactive oxygen species and can cause problems if it accumulates. 06:32 It's for this reason that the cell has an enzyme called catalase that breaks down hydrogen peroxide and prevents from having toxic effects. 06:39 The enzyme catalyzing this reaction is protoporphyrinogen oxidase. 06:44 We see the reaction going on here. 06:46 In the green box on the left, we see the central portion of the molecule and we see the result in structure on the right. 06:52 Now, at first glance, not a lot has happened but in fact six atoms of hydrogen have been extracted from the molecule on the left. 06:59 If look carefully, you'll see that there are several double bonds that have been created and a couple of very visible hydrogens that have been lost to make the final product of protoporphyrin IX. 07:10 Protoporphyrinogen oxidase is found in the inner mitochondrial membrane. 07:14 Variegate protoporphyria is caused by mutations in protoporphyrinogen oxidase. 07:20 The final step in the synthesis of heme is of course the insertion of the iron atom into the middle of the porphyrin group that we've been constructing so far. 07:30 We see on the left the protoporphyrin, or the protoporphyrin IX as it's also called, with an empty space in the middle. 07:36 On the right, we see the product, heme B which has had an atom of ferrous iron inserted in the middle. 07:41 The iron is inserted as we can see above. 07:43 This results in the loss of two protons. 07:47 There's electronic rearrangement that occurs in getting that iron in there and I won't go through that at this time. 07:53 The enzyme catalyzing this reaction is known as ferrochelatase. 07:57 And ferrochelatase is another enzyme that's found in the inner mitochondrial membrane. 08:02 Other hemes may require additional modifications and if you recall, those other modifications were reoccurring away from the rings outside of the structures that we see here. 08:10 They're fairly minor in the scheme of things. 08:13 I've been talking about the individual reactions and I've said that they occur in the mitochondrion or whatever, but I have not exactly shown you where they are. 08:20 So, I thought it would be helpful if I provided a figure that illustrated the individual reactions and the enzymes and where they are located. 08:27 In the central part of the figure, we see the mitochondrion with its matrix and with its inner membrane. 08:33 Outside that mitochondrion is the cytoplasm. 08:36 And we see that some of the reactions occur in the matrix. 08:39 The process actually starts there. 08:41 The molecules are moved out in the cytoplasm where some of the enzymes are located. 08:45 And then some of the molecules are moved back into the mitochondrial matrix where the other enzymes are located. 08:51 Ultimately what happens in this synthesis of heme is that heme is moved out of the mitochondrion to the inner mitochondrion membrane where you see the heme located. 08:59 It's there that it's attached the globins chains to make molecules like for example hemoglobin.
About the Lecture
The lecture Heme Synthesis by Kevin Ahern, PhD is from the course Amino Acid Metabolism.
Included Quiz Questions
Which of the following is true regarding the synthesis of heme?
- It starts with glycine and succinyl-CoA.
- It begins with a cytoplasmic enzyme.
- It begins with the incorporation of carbon dioxide.
- It begins with acetyl-CoA and glutamate.
- The first step is catalyzed by aminolevulinic acid decarboxylase.
Which of the following is true regarding the synthesis of porphobilinogen?
- Two glycines and two succinyl-CoAs would be required.
- A ring structure is destroyed.
- The enzyme is actually stimulated by heavy metals.
- Its formation requires decarboxylation.
- Porphobilinogen synthase is sensitive to cobalt ions.
Which of the following is true regarding the synthesis of hydroxymethylbilane?
- Ammonia is released during this process.
- Six glycines and six succinyl-CoAs would be required.
- Increased production results in a type of porphyria.
- A ferrous ion is inserted into its center immediately after it is synthesized.
Which of the following is true regarding uroporphyrinogen III?
- It is the first cyclic intermediate in heme synthesis.
- Its synthesis requires addition of water.
- It is found in excess in the condition of congenital erythropoietic porphyria.
- It is slowly converted to coproporphyrinogen III.
Which of the following is true regarding uroporphoryinogen III decarboxylase?
- It has the highest rate of catalysis of any known enzyme.
- It decarboxylates coproporphyrinogen III.
- It is a methodical enzyme, working very slowly.
- It requires four carbon dioxide molecules in the reaction it catalyzes.
Which of the following is true regarding the synthesis of protoporphyrinogen IX?
- The enzyme catalyzing its formation is in the mitochondrion.
- Two molecules of coproporphyrinogen III are consumed during its synthesis.
- The enzyme uses magnesium in catalysis.
- 2 molecules of oxygen are produced in the oxidative decarboxylation reaction it catalyzes.
Which of the following is not true regarding protoporphyrin IX?
- It is formed in a reduction reaction
- It is a precursor of heme and chlorophyll.
- It is ready to receive a ferrous ion into its center to form heme.
- It is formed in a reaction catalyzed by an inner mitochondrial enzyme.
Which of the following is true regarding heme?
- It requires electronic rearrangement for its final form.
- Its synthesis can start even if no iron-binding proteins are not present in the mitochondria.
- Ferrocatalase is required in the final step of its formation.
- It is formed as heme C and other hemes are made from it.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
it is really amazing lecture & has been organized well thank you
Thank you very much! Those extraordinarily awesome questions help me better understand the whole thing with details!