Playlist
Show Playlist
Hide Playlist
Glucose
-
Slides 04 MacromoleculesI CellBiology.pdf
-
Reference List Molecular and Cell Biology.pdf
-
Download Lecture Overview
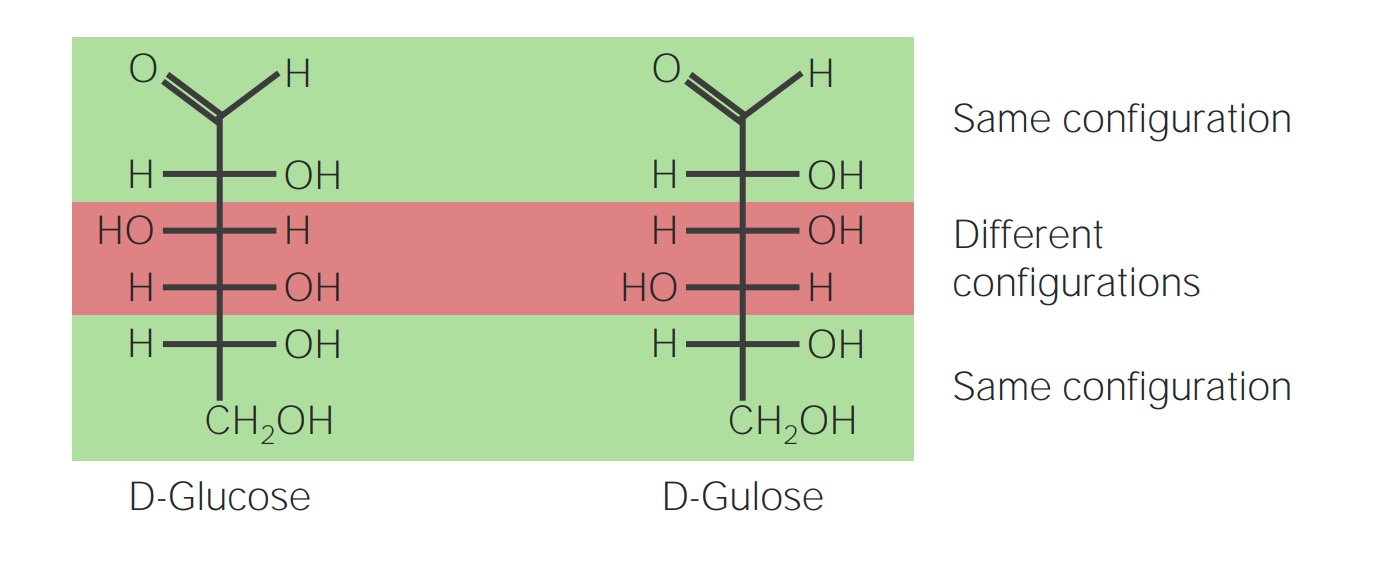
00:00 So now that we have an understanding of how things come together, how are monomers joined together to form polymers it's the same with every class of macromolecules. 00:10 Let's take a little bit deeper into carbohydrates. 00:14 Carbohydrates are molecules that are composed of monomers or monosaccharides. 00:21 Monosaccharides are simple sugars. 00:23 They come in the form of either three carbon sugars, there's R carbon backbone that's got hydrogen associated with it. 00:31 They could come in the form of five carbon sugars. 00:34 We'll see these when we look at ribose and deoxyribose and the structure of DNA and then we could have six carbon sugars ones we are familiar with are things like glucose and fructose and galactose. 00:47 These monomers can all come together to form polymer macromolecules. 00:53 We could have disaccharides or trisaccharides but mostly we deal with disaccharides which are two sugars coming together or polysaccharides which are multiple sugar units coming together. 01:05 In the case of disaccharides, we could look at something like glucose. 01:12 Glucose is a chain of six carbon atoms associated with their hydrogen so those are hydrocarbons. 01:19 Now, that molecule can fold, in solution it does fold into a ring structure where we see the terminal oxygen bind with the number five carbon in order to form that ring. 01:34 Now, something that will come to later on is that this could form in two different ways. 01:40 it can either fold in this direction and form alpha-glucose or it could fold in that direction and form beta-glucose. 01:48 The results here is that we have either an H-group up, an OH-group down or an OH-group up and an H-group down, and that is going to impact how these two molecules come together. 02:04 Again, we can see that there are multiple forms of C6-H12-O6 not only could they fold in a different direction to form the ring but they could also be a slightly different structural shape. 02:21 So we have the basic formula C6-H12-O6, they all have the same number of carbons, hydrogens and oxygens however, they could form a different arrangement with those carbons, hydrogens and oxygens. 02:37 We can have a structural isomer which is structurally quite different. 02:41 You can see here that we have a carbon oxygen double bond replacing one of the single bonds in which case it's quite a different molecule or we could have a stereo isomer in which one piece might be just reflected and it could be at any one of those carbons. 02:58 The point here is that this different structure will require a different enzyme to hydrolyze or break the sugar apart so each of this has a very specific fit to a certain enzyme that's responsible for breaking them down or even putting them together. 03:18 So, disaccharides are just two sugars together and they're generally responsible for storage and transports both within an organism and between different organisms so we could consume a disaccharide for example by consuming something like lactose. 03:41 So here let's look at alpha-glucose and dehydration synthesis forming alpha-glucoses together this is just one way the chain can form. 03:52 We look at alpha versus beta glucose and we could have alpha-glucose linked to fructose and we come up with a disaccharide that's called sucrose. 04:04 Here dehydration synthesis we lose an H from one end and OH from the other and they come together to form the disaccharide sucrose. 04:13 Lactose is a disaccharide that provides a great example of enzyme specificity. 04:19 There are two sugar monomers in there that we produce an enzyme for during our lactation years when we are feeding on milk up until about two years of age and in adulthood unless we're exposed to a diet very high in milk, generally, we don't produce the enzyme lactase that breaks down lactose. 04:41 So that's just an example of how the enzymes can be very specific to the type of sugar or the isomer of sugar that's involved in a polymer. 04:54 This is the case for many polymers whether we're looking at proteins or nucleic acids or lipids for that matter.
About the Lecture
The lecture Glucose by Georgina Cornwall, PhD is from the course The Macromolecules of Life.
Included Quiz Questions
Which of the following is true about fructose, glucose, and galactose?
- They all have the same chemical composition but different structures.
- Fructose is a stereoisomer of glucose.
- Galactose is a structural isomer of glucose.
- They all can be processed by the same enzyme.
- Only glucose is a carbohydrate.
Which of the following is TRUE about disaccharides?
- Lactose and sucrose are either stored or transported within and between organisms.
- Lactose is composed of fructose and glucose.
- Sucrose does not need an enzyme to be broken down.
- Sucrose is composed of two glucose molecules.
- Lactase, the enzyme that breaks down lactose, is mainly produced during late adulthood.
Customer reviews
2,5 of 5 stars
| 5 Stars |
|
0 |
| 4 Stars |
|
1 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
1 |
I belive she is very clear and resume complicated topics nicely.
not clear voice . . . . . . . . . . comparatively worse than other videos, others are good.