Playlist
Show Playlist
Hide Playlist
Epidermal Growth Factor Receptor (EGFR)
-
Slides HormonesSignalTransduction Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
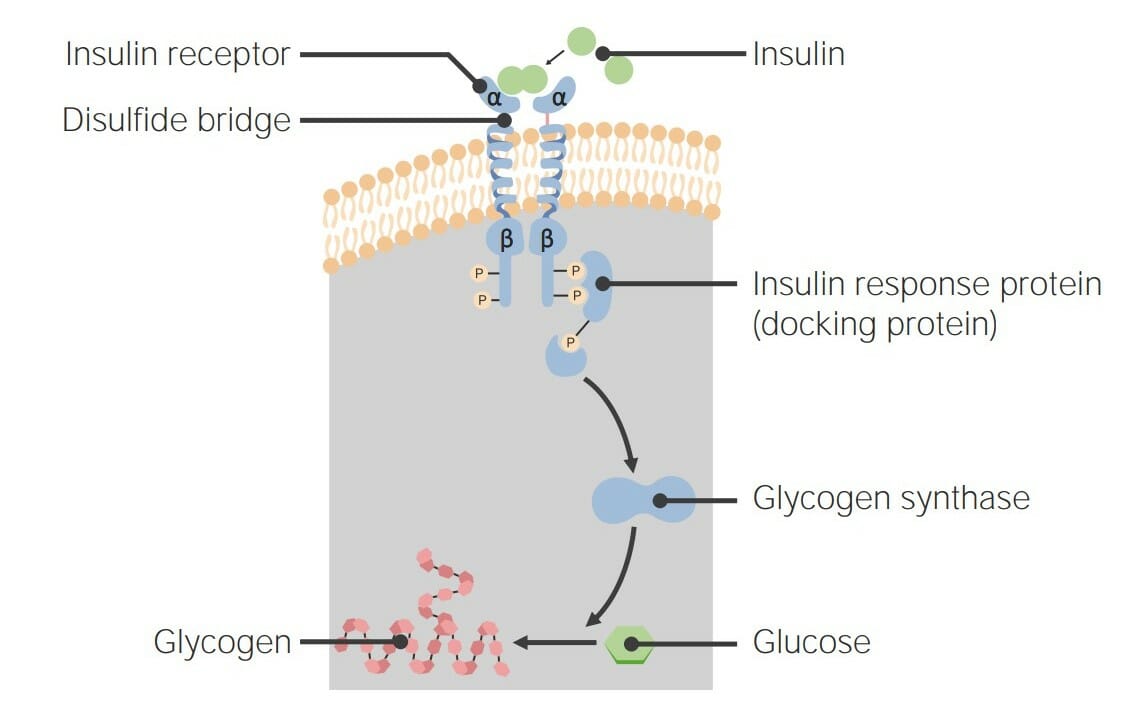
00:01 Das nächste Rezeptorsystem, über das ich sprechen möchte, ist das des epidermalen Wachstumsfaktor-Rezeptors oder EGFR (epidermal growth factor receptor). 00:07 Wie die anderen Rezeptoren, über die ich gesprochen habe, ist der epidermale Wachstumsfaktor-Rezeptor eine Rezeptor-Tyrosin-Kinase. 00:14 Es dimerisiert bei Bindung an epidermalen Wachstumsfaktor oder EGF. 00:19 EGF ist ein Protein, das am Wachstum und der Proliferation von Zellen beteiligt ist. 00:25 Und EGF hilft bei der Kontrolle dieses Gesamtprozesses. 00:31 Betrachten wir also diesen Signalweg aus der Nähe. 00:34 In dieser Abbildung ist die Bindung von EGF in grün dargestellt, auf dem äußeren Teil der Zelle an den EGF-Rezeptor. 00:42 Der Rezeptor hier hat sich dimerisiert und hält das EGF in der Zellmembran fest. 00:47 Wir können einige akzessorische Proteine sehen, die hinzugekommen sind und die begonnen haben, den Signalkomplex im Inneren der Zelle zu bilden. 00:55 Dazu gehört ein Protein namens GRB2. 00:57 Und GRB2 bindet an Phosphoytyrosine, die sich auf der zytoplasmatischen Seite der Zelle, auf dem EGF-Rezeptor, befinden. 01:07 Die Autophosphorylierung, die ich zuvor beschrieben habe, hat hier stattgefunden. 01:11 Und dieses GRB2 hat eine SH2-Domäne, die diese Phosphoproteine erkennt und an sie bindet. 01:17 GRB2 bindet an ein anderes Protein, das als SOS bekannt ist, was für unsere Zwecke hier unbedeutend ist. 01:23 Wichtig ist jedoch das nächste Protein das bindet und als RAS bekannt ist. 01:27 RAS ist ein Protein, das die Entscheidung der Zelle, sich zu teilen, sehr stark beeinflusst. 01:34 RAS ist ein Protein, das dem G-Protein sehr ähnlich ist, das ich zuvor beim beta-adrenergen Rezeptor beschrieben habe. 01:40 Es hat eine ähnliche Größe und bindet auch an Guanin-Nukleotide. 01:46 Das Guanin-Nukleotid, an das es hier bindet, ist GDP. 01:50 Und wenn es aktiviert wird, was hier in diesem Prozess geschieht, wird das GDP durch GTP ersetzt und RAS ist nun aktiv und erledigt seine Aufgabe. 02:00 Und aktives RAS bereitet die Zelle auf die Teilung vor. 02:05 Nun, dieser Weg hat noch mehr zu bieten; RAS geht wiederum zu einem anderen Protein namens RAF, das eine Kinase ist und aktiviert es. 02:14 RAF setzt ein Phosphat an ein anderes Protein, das als MEK bekannt ist und eine Kinase ist, die ein Phosphat an ein anderes Protein, bekannt als MAPK hängt, das natürlich wiederum eine Kinase ist, die Phosphate auf andere Proteine überträgt, wie wir hier sehen können. 02:27 Nun, die Reihenfolge ist für unsere Zwecke nicht von Bedeutung. 02:30 Wichtig ist jedoch, was im Gesamtprozess passiert. 02:34 Diese Kaskade von Phosphorylierungen, die stattfindet führt dazu, dass eine Reihe von verschiedenen Proteinen aktiv werden und das wirkt sich ganz unten auf einige Proteine aus, die als Transkriptionsfaktoren bekannt sind. 02:47 Diese Transkriptionsfaktoren werden durch die Zugabe von Phosphat aktiviert. 02:51 Und sie wandern in den Zellkern, wie Sie hier sehen können, um die Genexpression zu ko-aktivieren. 02:58 Die Gene, die sie aktivieren, werden die Zelle zur Teilung anregen. 03:02 Der epidermale Wachstumsfaktor hat also den Prozess der Zellteilung begünstigt. 03:06 Nun, bevor ich weitermache, muss ich ein interessantes Wort über RAS sagen. 03:10 RAS ist eigentlich eine Familie von verwandten Proteinen, es ist nicht nur eines, sondern es gibt mehrere. 03:16 Jedes dieser Proteine ist monomer, das unterscheidet sich von dem, was wir vorher bei den G-Proteinen gesehen haben: sie hatten die Heterotrimere, die wir mit dem β-adrenergen Rezeptor assoziiert haben. 03:26 RAS-Proteine können Guanin Nukleotide binden, wie wir gesehen haben. 03:30 Und dieses menschliche RAS, das ist hier an ein GDP gebunden. 03:35 RAS tauscht GDP gegen GTP aus, wenn es durch den Signalisierungskomplex aktiviert wird, den ich vorhin gezeigt habe. 03:43 Wie die α-Untereinheit des β-adrenergen Rezeptors ist auch das RAS ein schlechtes Enzym. 03:50 Es spaltet GTP sehr langsam zu GDP ab, was bedeutet dass sich RAS mit der Zeit selbst abschaltet. 03:59 Nun das ist gut, denn man will nicht, dass eine Zelle ständig eingeschaltet ist und sich ständig teilt, denn das wird als Krebs bezeichnet. 04:07 Wie wir bereits sagten, ist es für eine Zelle wichtig, ein Signal ausschalten zu können, genauso wie es wichtig ist, ein Signal einzuschalten. 04:13 Und das ist besonders wichtig für RAS, wie wir gesehen haben. 04:16 Wie ich bereits sagte, ist RAS eine schlechte GTPase. 04:19 Es wandelt GTP in GDP um und schaltet sich selbst aus. 04:24 Was passiert, wenn RAS sich nicht selbst abschalten kann? Das passiert manchmal. 04:28 Und leider passiert es zu häufig. 04:30 Dabei handelt es sich um sogenannte Punktmutationen oder Veränderungen einzelner Basen innerhalb der RAS-Kodierungssequenzen, die, wenn sie sich verändern, die Fähigkeit von RAS, GTP zu spalten, beeinträchtigen können. 04:42 Wenn sie die Fähigkeit von RAS, GTP zu spalten, hemmen, wird RAS immer im eingeschalteten Zustand belassen. 04:48 Nun, Sie haben auf der letzten Folie gesehen, was passiert, wenn die Zelle ständig eingeschaltet bleibt: Sie teilt sich unkontrolliert weiter und das kann zu Krebs führen. 04:56 Und RAS ist verwickelt in zahlreiche menschliche Krebsarten. 05:00 Jetzt müssen auch andere Dinge ausgeschaltet werden. 05:02 Die RTK, d.h. der EGF-Rezeptor, über den ich hier speziell gesprochen habe, muss ebenfalls ausgeschaltet werden. 05:08 Wie wird er ausgeschaltet? Nun, wie der β-adrenerge Rezeptor, wird er durch einen Prozess, bekannt als Endozytose, in die Zelle internalisiert, und ist somit nicht mehr Teil der Signalisierungsprozesse. 05:20 Die Phosphatasen, die stimuliert werden in den Schemata, die ich zuvor gezeigt habe, sind Proteine, die anderen Proteinen Phosphate entziehen. 05:27 Und diese ganze Kaskade, die ich vorhin gezeigt habe, bestand aus einer ganzen Reihe von phosphorylierten Proteinen. 05:33 Eine Phosphoproteinphosphatase, die auf sie einwirkt, inaktiviert den gesamten Weg mit einer einzigen Aktion. 05:40 Das ist ziemlich cool. 05:42 Sie könnten sich nun fragen, wie es kommt, dass die Phosphoproteinphosphatase selbst inaktiviert wird? Und es stellt sich heraus, dass sie durch einen interessanten Phosphorylierungsprozess inaktiviert wird. 05:52 Ich wollte mir eine Minute nehmen und Ihnen das zeigen. 05:54 Auf der rechten Seite des Bildschirms sehen Sie die Phosphoproteinphosphatase in grün, die an ein Protein namens Gm gebunden ist; das ist kein G-Protein. 06:03 Es ist nur ein Muskelprotein namens Gm. 06:05 In der Form, die Sie oben sehen, ist es aktiv. 06:08 Eine Phosphorylierung des Gm bewirkt jedoch, dass das Gm die Phosphoproteinphosphatase freisetzt. 06:16 Dadurch wird die Phosphoprotein- phosphatase weniger aktiv. 06:20 Sie sehen dort draußen auch einen Hemmstoff schwimmen. 06:23 Der Inhibitor selbst bindet nicht an die Phosphoproteinphosphatase und hemmt sie. 06:28 Vielmehr wartet es darauf, dass der Inhibitor selbst phosphoryliert wird. 06:34 Wenn der Inhibitor phosphoryliert wird, bindet die Phosphoproteinphosphatase an ihn und wird dann vollständig inaktiviert. 06:41 So wird es also inaktiviert. 06:43 Die Frage ist dann, wann wird es inaktiviert? Und es wird durch Wirkung der Proteinkinase A inaktiviert. 06:52 Denken Sie nun über diese wechselseitige Regulierung nach. 06:55 Diese wechselseitige Regulierung führt dazu, dass eine Reihe von Enzymen bei der Bindung eines Hormons aktiv und eine andere inaktiv wird, um sie dann mit dem anderen Hormon umzukehren. 07:04 Im Falle des Epinephrins zum Beispiel, über das ich sprach, stimulierte es Proteine, die für den Abbau von Glykogen wichtig waren. 07:12 Und Insulin war wichtig für die Stimulierung von Proteinen, die Glykogen herstellen. 07:16 Wir sehen, dass sich diese wechselseitige Regulierung bis hin zum Ausschalten von Proteinen wie der Phosphoproteinphosphatase erstreckt. 07:23 Ziemlich cooler Prozess.
About the Lecture
The lecture Epidermal Growth Factor Receptor (EGFR) by Kevin Ahern, PhD is from the course Hormones and Signal Transduction. It contains the following chapters:
- Epidermal Growth Factor Receptor (EGFR)
- RAS
Included Quiz Questions
Which of the following are true about the Epidermal Growth Factor Receptor (EGFR)? Select all that apply.
- It is involved in the growth and proliferation of cells.
- It is located in the nucleus.
- It dimerizes upon binding EGF.
- It is a type of receptor tyrosine kinase.
- It can stimulate growth in the absence of hormones.
Which statement regarding RAS signaling is true?
- It is focused on stimulating transcription.
- It requires that RAS be a dimer to function.
- It gets a phosphate put onto its GDP to make GTP.
- It inactivates RAF.
Which statements regarding phosphatases are true? Select all that apply.
- They can inactivate kinase enzymes.
- They activate kinase enzymes by phosphorylating them.
- They are involved in regulating the cascade induced by RAS.
- They can be inactivated by a phosphorylated inhibitor.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |