Playlist
Show Playlist
Hide Playlist
β-adrenergic Receptor Signaling
-
Slides HormonesSignalTransduction Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
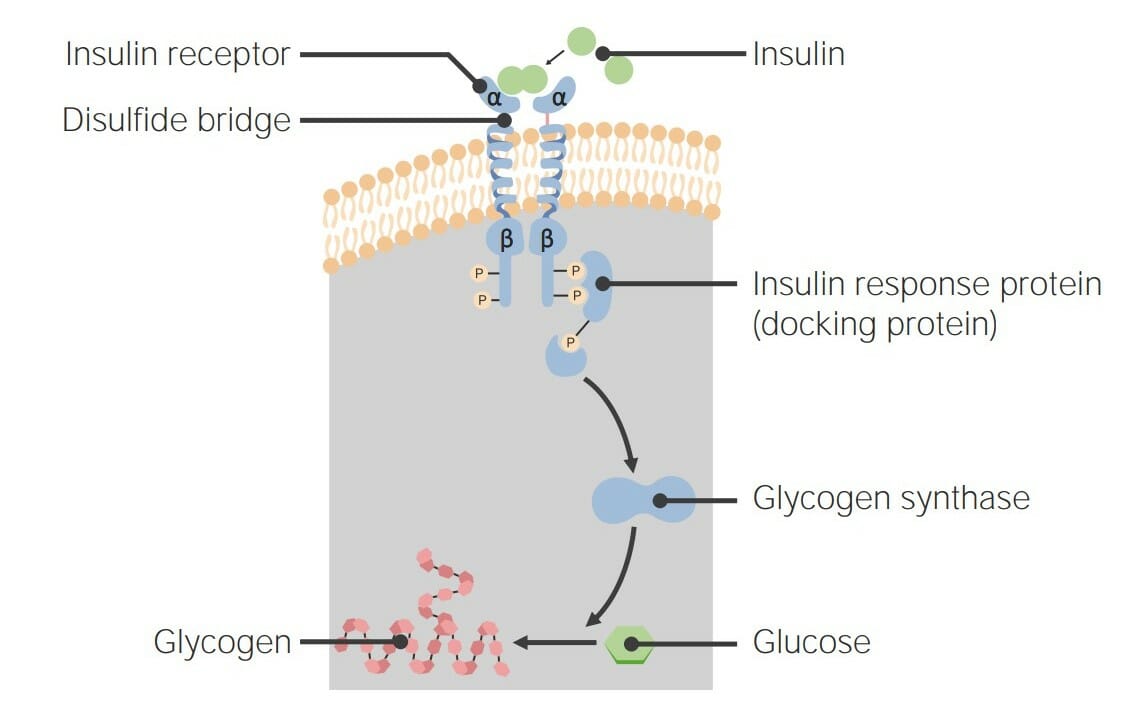
00:01 Wenn wir uns nun ansehen, was mit diesem System geschieht, das übrigens als β-adrenerger Rezeptor bekannt ist. 00:07 Was passiert in diesem System? Wir können tatsächlich den den gesamten Prozess sehen. 00:11 Es gibt das Hormon, das sich an den Rezeptor bindet, es gibt die Aktivierung des G-Proteins, wobei die α-Untereinheit ein GTP erhält. 00:19 Die Wechselwirkung der α-Untereinheit mit der Adenylatcyclase. 00:23 Die Bildung von zyklischem AMP. 00:25 Die Aktivierung der Proteinkinase A, die Aktivierung der Phosphorylase-Kinase. 00:32 Die Inaktivierung der Glykogensynthase. 00:35 Die Aktivierung von Glykogenphosphorylase-a. 00:37 Und der Abbau von Glykogen. 00:40 Dieser Prozess läuft sehr schnell ab. 00:44 Alles, was wir hier sehen ist eine enzymatische Reaktion. 00:46 Und Enzyme arbeiten sehr, sehr schnell. 00:49 Sie sehen, dass bei diesem Weg keine Bewegung in den Zellkern stattfindet. 00:54 Dies ist wichtig, weil Bewegung in den Zellkern und die Aktivierung der Genexpression ein sehr langsamer Prozess ist. 01:02 In unseren Zellen dauert es etwa 24 Stunden, bis das passiert. 01:05 Nun, das ist wichtig, wenn man sich zum Beispiel in einer dunklen Gasse befindet, denn, stellen Sie sich vor, dass Sie jemand verfolgt. 01:10 Sie müssen Energie haben und Sie müssen diese Energie jetzt haben. 01:14 Sie können nicht 24 Stunden auf die Genexpression warten, um etwas herzustellen, aus dem man Glukose gewinnen kann. 01:20 Dieser Signalweg, der die Aktivität von Enzymen reguliert und nichts mit der Genexpression zu tun hat, ist ein Beispiel das sehr, sehr schnell funktioniert. 01:31 Wenn wir nun darüber nachdenken, ein System einzuschalten, wie das, das ich hier beschrieben habe, ist das ziemlich einfach. 01:36 Aber wir denken, dass die Zellen das System nicht ständig eingeschaltet haben können, denn wenn sie dieses System ständig eingeschaltet ließen, würden sie auch ständig ihr Glykogen abbauen. 01:45 Sie würden kein Glykogen mehr haben. 01:46 Und Glykogen ist ein ziemlich wichtiges Speichermolekül für Energie. 01:50 Genauso wie sie also in der Lage sein müssen, den Glykogenabbau schnell einschalten zu können, müssen sie ihn auch schnell abschalten können, wenn der Bedarf an Glukose aus dem Glykogenabbau nachlässt. 02:02 Es gibt also mehrere Dinge, die in diesem Prozess deaktiviert werden müssen. 02:06 Ich werde Sie nun schrittweise durch den Prozess der Deaktivierung des β-adrenergen Rezeptors führen. 02:11 Der Rezeptor selbst muss inaktiviert werden. 02:14 Das G-Protein, das ich mit der α-Untereinheit beschrieben habe, muss deaktiviert werden. 02:18 Das zyklische AMP, das kleine Molekül, muss entweder zerstört werden oder auf irgendeine Weise versteckt, so dass es seine Wirkung nicht entfalten kann. Die Proteinkinase A muss ihre regulatorischen Untereinheiten zurückgewinnen. Die Phosphorylase-Kinase muss sein Phosphat verlieren. Glykogenphosphorylase A muss inaktiviert werden. Und wenn das alles passiert, muss die Glykogensynthase, die Synthese von des Enzyms, das an der Glykogensynthese beteiligt ist, aktiviert werden. All diese Dinge müssen also passieren und die Umkehrung dieses Prozesses. Nun, schauen wir uns an, wie das passiert. Wir beginnen mit dem Rezeptor. 02:52 Der Rezeptor wird hier so dargestellt, wie wir ihn zuvor gesehen habe. 02:54 Der Rezeptor ist so dargestellt, dass der äußere Teil der Zelle oben und der innere Teil unten ist, wo das G-Protein mit ihm interagiert. 03:04 Bei der Inaktivierung des Rezeptors kommt es unter anderem zu einer Phosphorylierung des Rezeptors. 03:11 Es wird ein Phosphat daran angehängt. 03:13 Und dies geschieht durch die Wirkung eines Proteins, das als G-Protein-Rezeptor-Kinase bekannt ist. 03:19 Sie können die Hinzufügung des Phosphat an der Unterseite des Rezeptors sehen. 03:23 Dieses Phosphat ist das Ziel für die Bindung eines Proteins, das als Arrestin bekannt ist. 03:28 Jetzt sieht Arrestin das Phosphat, ergreift es und deckt es zu. 03:32 Durch die Anwesenheit von Arrestin wird verhindert, dass das G-Protein mit seinem Rezeptor interagiert. 03:39 Dadurch wird der Rezeptor blockiert und daran gehindert, weitere Signale in die Zelle zu leiten. 03:45 Es begünstigt auch den Prozess der Endozytose, d.h. das Hereinziehen des Rezeptors in die Zelle, so dass die Zelle ihn entweder abbauen oder etwas anderes mit ihm machen kann. 03:55 Wir haben also den Rezeptor als das Ergebnis der hier beschriebenen Aktion inaktiviert. 04:01 Als Nächstes denken wir darüber nach - und nebenbei bemerkt, ich beschreibe das in einer Reihenfolge. 04:04 In den meisten Fällen sind sie nicht mit einer Reihenfolge verbunden. 04:07 Aber ich werde über sie in der Art sprechen, wie sie während des gesamten Signalisierungsprozesses passieren. 04:13 Der nächste Schritt ist, dass das G-Protein selbst inaktiviert werden muss. 04:17 Und das stellt sich als ein wirklich interessantes Phänomen heraus. 04:20 Wir sehen hier zunächst, dass das G-Protein nicht mit dem Rezeptor interagieren kann. 04:23 Aber wir erinnern uns, dass Zellen Hunderte von Kopien eines Rezeptors haben und wir sehen uns hier nur eine an. 04:29 Nur weil wir einen Rezeptor blockieren, bedeutet das nicht, dass die anderen Rezeptoren nicht verfügbar sind. 04:35 Das bedeutet, dass das G-Protein selbst deaktiviert werden muss. 04:39 Und dieser Deaktivierungsprozess ist etwas, das es selbst durchführt, was ziemlich cool ist. 04:44 Wie kann das passieren? Es hat sich herausgestellt, dass die α-Untereinheit des G-Proteins ein sehr schlechtes Enzym ist. 04:52 Ja richtig, das habe ich gesagt. 04:52 Es ist ein sehr schlechtes Enzym. 04:55 Was bedeutet das? Es bedeutet, dass es eine Reaktion katalysiert, aber es katalysiert sie nicht sehr gut oder sehr schnell. 05:02 Die Reaktion, die es katalysiert, ist die Spaltung von GTP. 05:06 Und bei der Zerlegung von GTP, ist das Produkt GDP. 05:10 Und warum ist das wichtig? Erinnern Sie sich, als das GDP an die α-Untereinheit gebunden war, befand sie sich nicht mehr im aktiven Zustand. 05:17 Sie interagiert nicht mehr mit der Adenylatcyclase. 05:20 Die α-Untereinheit hat sich also bei dieser Reaktion selbst ausgeschaltet. 05:24 Und dass es ein sehr ineffizientes oder ein sehr schlechtes Enzym ist, bedeutet dass es sich nicht selbst abgeschaltet hat, sobald es das GTP bekam. 05:33 Wenn es ein gutes Enzym wäre, würde es das GTP sofort abbauen und es würde kein Signal übertragen. 05:38 Dieser Prozess der Aufspaltung des GTP kann ein paar Sekunden bis zu ein paar Minuten dauern. 05:44 Genug Zeit, um das Signal zu übermitteln, aber nicht um zuzulassen, dass sich das Signal weiter ausbreiten kann. 05:48 Ziemlich cool. 05:51 Sobald das GDP wieder in der α-Untereinheit ist, wie wir hier sehen, können die β- und γ-Untereinheiten wieder mit ihr interagieren. 05:58 Und dieser Komplex geht dann schließlich zu einem anderen β-adrenergen Rezeptor oder GPCR und wartet auf das nächste Signal. 06:07 Das nächste Molekül, dessen Eliminierung ich beschreibe, ist das zyklischem AMP. 06:12 Sie erinnern sich, dass zyklisches AMP notwendig war um die Proteinkinase A zu aktivieren. 06:17 Zyklisches AMP wird durch ein Enzym, der sogenannten Phosphodiesterase, abgebaut. 06:21 Jetzt ist die Zelle voll von Phosphodiesterase. 06:23 Phosphodiesterase ist normalerweise vorhanden und in der Regel in einer aktiven Form. 06:30 Das zyklische AMP, wenn es einmal gemacht wurde, bleibt normalerweise auch nicht für sehr lang, denn es wird von der Phosphodiesterase gefunden und zu gespalten. 06:40 Nun, als AMP kann es nichts ausrichten, daher ermöglicht die Phosphodiesterase es, dass zyklisches AMP schnell abgebaut werden kann. 06:50 Nun, zyklisches AMP war natürlich notwendig für für die Aktivierung der Proteinkinase A. 06:54 Und Sie sehen dort die grüne Form. 06:55 Ohne das Vorhandensein von zyklischem AMP können also die regulatorischen Untereinheiten nun zurückkommen und das wenige zyklische AMP ersetzen, das mit der Proteinkinase A assoziiert war und sie stellen die an die regulatorische Untereinheit gebundene inaktive Proteinkinase A wieder her. 07:11 Die Proteinkinase A wurde somit ausgeschaltet. 07:16 Gut, der nächste Prozess im Schema ist die Entfernung von Phosphaten. 07:20 Und es stellt sich heraus, dass dies tatsächlich der einfachste Schritt ist, der passiert. 07:24 Es gibt ein Enzym, das als Phosphoprotein-Phosphatase bekannt ist. 07:27 Und Phosphoproteinphosphatase ist ein Enzym, das durch die Zugabe von Insulin an der Außenseite einer Zelle stimuliert wird. Insulin veranlasst dieses Protein, aktiv zu werden und dieses Protein entfernt Phosphate von anderen Proteinen. Nun, Wir können uns vorstellen, was hier passieren wird. 07:45 Die Entfernung von Phosphat aus der Phosphorylase-Kinase bewirkt, dass die Phosphorylase-Kinase von der aktiven in die inaktive Form übergeht. 07:55 Wir sehen, dass die Entfernung des Phosphats aus der Glykogenphosphorylase-a die Umwandlung in die Form der Glykogenphosphorylase-b verursacht, die ebenfalls inaktiv ist. 08:03 Und der Entzug des Phosphats aus der Glykogensynthase bewirkt, dass sie sich in die grüne Form umwandelt, die die aktive Form der Glykogensynthase ist. 08:12 Mit diesem letzten Schritt wurden nun alle die Prozesse, die während der Bindung des Epinephrins aktiviert wurden, umgekehrt. Es gibt noch eine andere Sache, zu der ich etwas sagen möchte. Und diese eine Sache, über die ich etwas sagen möchte ist, dass ich Sie zu dieser Folie hier zurückbringe. Diese Folie, an die Sie sich noch erinnern, zeigte vorhandenes, aktives zyklisches AMP. Und während zyklisches AMP vorhanden war, waren all diese anderen Proteine aktiv sind und die Glykogensynthase inaktiv. Aber warum komme ich zurück zu dieser Folie? Ich kehre zu dieser Folie zurück, denn erinnern Sie sich: Die Phosphodiesterase baut zyklisches AMP ab. Eine wirklich coole Tatsache ist, dass die Phosphodiesterase, die zyklisches AMP zu AMP abbaut, durch Koffein gehemmt wird. Die morgendliche Tasse Kaffee, die Sie getrunken haben und von der Sie sagten, dass sie Ihnen einen kleinen Kick gegeben hat, das kam von der Tatsache, dass Sie mehr Glukose ausschütten, wenn zyklisches AMP in Ihren Zellen vorhanden ist. Der kleine Rausch, den Sie hatten, war also ein bisschen Zucker, der aus der Hemmung der Phosphodiesterase durch Koffein stammt.
About the Lecture
The lecture β-adrenergic Receptor Signaling by Kevin Ahern, PhD is from the course Hormones and Signal Transduction. It contains the following chapters:
- β-adrenergic Receptor Signaling
- G-protein Inactivation
- cAMP
Included Quiz Questions
Which actions occur in turning off the glycogen breakdown hormonal regulation pathway?
- cAMP is broken down by phosphodiesterase.
- Arrestin favors exocytosis.
- Caffeine inhibits glycogen phosphorylase.
- The α-subunit of the G-protein turns itself off.
- Phosphoprotein phosphatase converts GTP to GDP.
What masks the β-adrenergic receptor?
- The arrestin protein after phosphorylation on its intracellular part by G-protein receptor kinase enzyme.
- The G-protein receptor kinase, under the guidance of arrestin protein.
- The phosphate group on its extracellular domain part.
- The cAMP molecule on its intracellular domain sites.
- The GDP-αβγ molecular entity under the guidance of G-protein.
What does the alpha subunit of the G-protein do?
- It breaks down GTP.
- It synthesizes ATP.
- It breaks down GDP.
- It digests adenylate cyclase.
- It autodigests itself.
Which enzyme breaks down the cyclic AMP molecule?
- Phosphodiesterase
- G-protein receptor kinase enzyme
- Phosphatase
- Protein kinase A
- Phosphorylase kinase
Customer reviews
3,7 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
1 |
Thank you so much This video helped me so much en my sesions of study
Thank you Dr. Ahern for this very comprehensive lecture. Going through the biochem book makes this topic very complicated. It is amazing how you can simplify this topic to get to the point and what we actually need to understand.
the lectures is shallow; i think that more details are needed. it is ineffectual for the level required from the biochemestry course of the Med University of Florence. cordially gioia