Playlist
Show Playlist
Hide Playlist
Approach to Acid Base Status: Step 1 and 2 – Laboratory Diagnostics
-
Slides DiagnosticsAcidosisAlkidosisStep1-2 RespiratoryPathology.pdf
-
Reference List Pathology.pdf
-
Download Lecture Overview
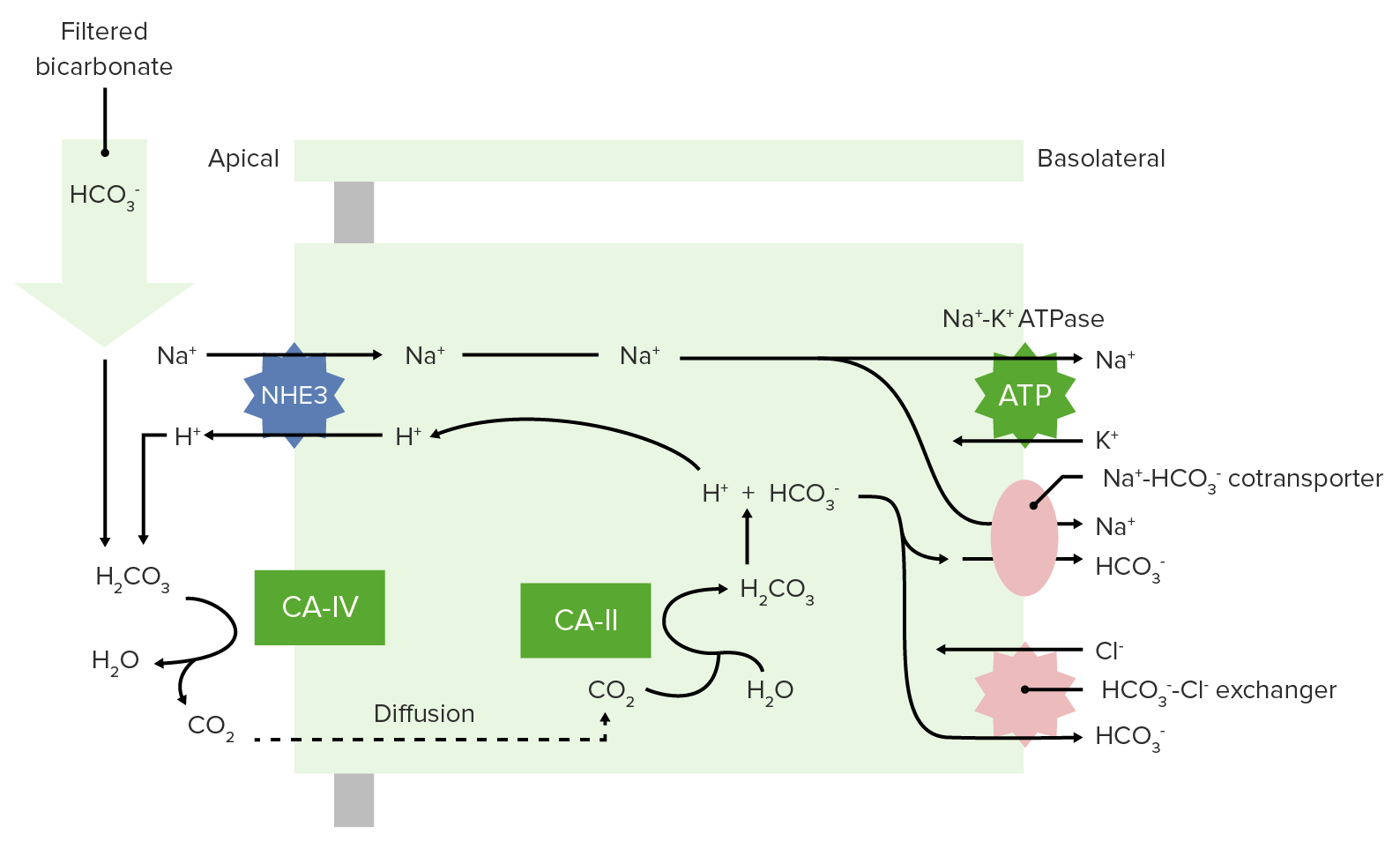
00:01 An organize fashion to approach all these one more time – pH less than 7.35 puts in the realm of acidosis, pH that’s greater than 7.45 puts in an environment of alkalosis. 00:13 What's your next question? What's my primary? Well, if you find your PCO2 to be above 40, then this has to be respiratory acidosis. 00:24 Tell me about the breathing pattern – good, hypoventilation; retaining carbon dioxide. 00:30 Let's do PCO2 less than 40. Hmm, this cannot be respiratory acidosis – so by default what is it? [Inaudible 00:00:38] metabolic acidosis, so therefore you expect your bicarb to be? Good, less than 20 maybe it’s 18, therefore this is compensation. 00:47 Give yourself an examples such as DKA, both moving in the same direction. 00:52 With alkalosis, what's your primary? If your PCO2 is greater than 40 it cannot be respiratory alkalosis. 01:01 Once again, by default, has to be metabolic alkalosis and you expect your increase in bicarb to be the primary thus the compensation would be an increase in carbon dioxide – same direction, good. 01:13 And what about PCO2 less than 40 – then this would be a respiratory alkalosis, And tell me quickly, what about compensation? Days, thus we have to divide into acute and chronic. 01:25 Now, this whole thing that I've been making a big deal about acute and chronic, what is this all about? The next step of management in some of these issues are going to be – if it’s acute and chronic, is the degree of compensation adequate or not adequate to take care of the primary disorder? Keep that in mind as we move on to the next few topics.
About the Lecture
The lecture Approach to Acid Base Status: Step 1 and 2 – Laboratory Diagnostics by Carlo Raj, MD is from the course Pulmonary Diagnostics.
Included Quiz Questions
Which of the following is the correct choice based on the following values? pH = 7.18 pCO2 = 39 mmHg HCO3 = 12 mEq/L
- Metabolic acidosis
- Metabolic alkalosis
- Respiratory acidosis
- Respiratory alkalosis with a concomitant metabolic alkalosis.
- Respiratory acidosis with a concomitant metabolic acidosis.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
It is clear, well explained and very useful. Totally recommended.