Playlist
Show Playlist
Hide Playlist
Analogs: Adenosine, Deoxycytidine, Guanosine/Deoxyguanosine, Thymidine, Deoxyuridine
-
Slides NucleotideMetabolism Biochemistry.pdf
-
Reference List Biochemistry.pdf
-
Download Lecture Overview
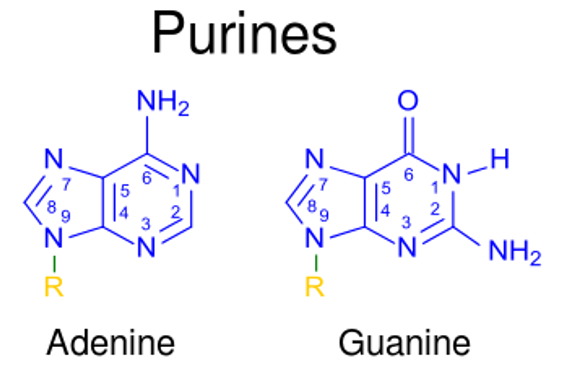
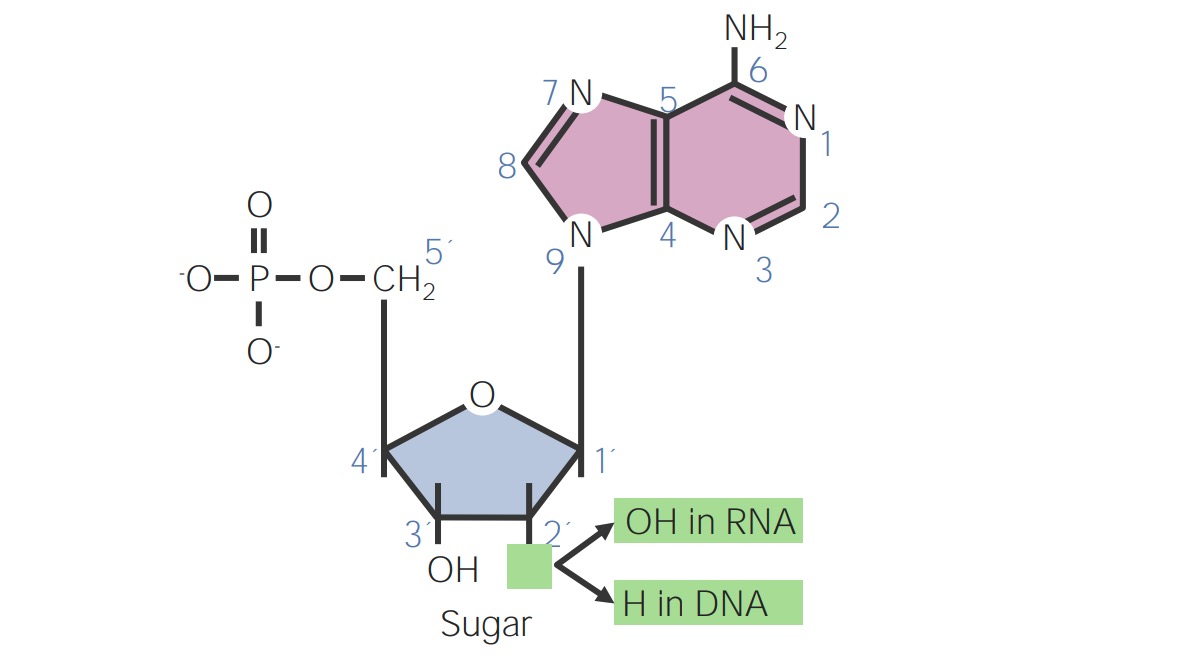
00:00 Adenosin ist natürlich das Molekül, das die konkurrierende Version von Desoxyadenosin ist. Die Unterschiede sehen Sie hier. In der Position Nummer 2 befindet sich ein Hydroxyl anstelle eines Wasserstoffs. Diese Verbindung BCX4430, mit seinem komplizierten Namen, ist ein Derivat von Adenosin. Wir können die leichte Veränderung in der Struktur der Base sehen, die an den Ribosering gebunden ist. Diese Unterschiede werden hier in den grünen Quadraten angezeigt. BCX4430 ist ein kompetitiver Inhibitor von ATP, wenn es phosphoryliert wird. Als kompetitiver Inhibitor des ATP kann es ein paar Dingen. Zum einen kann es die Synthese einer RNA stoppen. Zum anderen kann es die Zelle stören dieses Molekül als Triphosphat zur Energiegewinnung zu nutzen. Dieses BCX4430 ist ein starkes antivirales Mittel und wird zur Behandlung von Menschen eingesetzt, die mit dem Ebola-Virus infiziert sind. Deoxycytidin, wie hier zu sehen ist, und Derivate davon, ara-C, wie hier zu sehen ist, haben ähnliche Eigenschaften wie wir zuvor gesehen haben. Also wie das Ara-A, das eine Arabinose an dieser Position hat, hat Cytarabin eine Arabinose als Zucker an seiner Stelle, wie hier zu sehen ist. Dieses Molekül wird in der Chemotherapie verwendet, weil es die Fähigkeit der Zellen nutzt dieses als Cytidin-Nukleotid für den Aufbau einer Nukleinsäure zu verwenden. Zalcitabin oder ddC ist hier zu sehen. Und nun beginnen wir eine gewisse Ähnlichkeit der Muster zu erkennen, die zwischen den Derivate besteht. Dieser Verbindung fehlt wiederum eine Hydroxylgruppe an Position 3 und wie wir vorhin gesehen haben, bedeutet das Fehlen von Hydroxyl an Position 3, dass dieses Molekül eine Terminator der Kette bei der Synthese der DNA sein wird. Zalcitabin ist auch als ddC bekannt. ddC ist ein antiviraler Wirkstoff, der häufig bei den 3-Wirkstoff-Cocktails zur Behandlung von HIV verwendet wird. Hier ist ein Beispiel: Emtricitabin hat die Struktur, die Sie hier sehen können, und Sie sehen, dass es nicht einmal eine Menge von Dingen hat, die Sie im Verhältnis zum Desoxycytidin erkennen. Das ist jedoch die Art und Weise, wie die Infektionserreger es sehen. Wir sehen in erster Linie das Fluor, das hier anders ist und wir sehen den Zucker, der erstaunlicherweise auf dem Kopf steht. Dieser Wirkstoff hat einige wirksame antivirale Aktivitäten und wird auch in der HIV-Behandlung eingesetzt. Ein weiteres seltsames Zuckerderivat ist Lamivudin, das als 3TC bekannt ist. 3TC hat einen modifizierten Zucker, wie hier gezeigt ist und es ist auch ein starkes antivirales und Anti-HIV-Mittel. Es wird auch in dem 3-Medikamenten-Cocktail zur Behandlung von HIV-Patienten verwendet. Guanosin und Deoxyguanosin-Derivate sind ebenfalls wirksame Verbindungen, die zur Behandlung dieser Infektionen. 03:03 Hier ist Abacavir abgebildet und wir sehen, dass es ein paar merkwürdige Dinge an sich hat. 03:08 Erstens fehlt das Hydroxyl in Position 3 und zweitens, was Sie vielleicht nicht bemerkt haben, hat es ebenfalls eine Doppelbindung an dieser Stelle, was bedeutet, dass es sich um eine sehr ungewöhnliche Zuckerstruktur handelt. 03:19 Außerdem sehen wir ganz oben auf diesem Guanosin-Derivat, dass es Cyclopropan hat, die eine sehr reaktive und sehr seltsame Struktur hat. 03:33 Abacavir wird in erster Linie als Mittel gegen HIV eingesetzt. Acyclovir hat eine sehr merkwürdige Struktur, weil der untere Teil des Zuckers fehlt. Man sieht den unteren Teil des Zuckers überhaupt nicht, aber ss wird zur Behandlung von Herpes und anderen Viren eingesetzt. Entecavir hat wiederum ein paar Eigenheiten in der Struktur. Sie können sehen, dass es erstens ein doppelt gebundenes Kohlenstoffatom an der Position am oberen Ende des Ringes hat. Dies wird zur Behandlung von Hepatitis B verwendet. Auch Thymidinderivate sind wichtig. Hier ist eines mit dem Namen Stavudin, bei dem, wie auf der vorherigen Folie, das Hydroxyl an Position 3 ist und die Doppelbindung am unteren Ende ist. Sie können diesen Unterschied hier sehen. Stavudin wird als Anti-HIV-Mittel verwendet und ist auch ein antivirales Mittel, das das Wachstum von bestimmte Viren hemmt. Bei Telbivudin, wie wir hier sehen, gibt es einige Mischungen und die Übereinstimmung tritt auf, weil alles umgedreht ist. Die Basis ist auf der falschen Seite des Zuckers und auch das Hydroxyl ist im Wesentlichen in die falsche Richtung gedreht. Wir können diesen Unterschied sehen. 04:40 Diese Verbindung wird zur Behandlung von Hepatitis B verwendet. Zidovudin auch bekannt als AZT, wird zur Behandlung von HIV verwendet und hat eine seltsame Struktur an Position Nummer 3. 04:54 Es fehlt dort nicht völlig etwas, aber es hat diese Gruppe von Stickstoffen, die dort gebunden ist. 04:59 Dieses wird teilweise als Substrat vom HIV-Virus verwendet, aber auch hier kann es sich nicht ausbreiten, sobald es in eine wachsende DNA-Kette eingebaut wurde. Wie ich bereits erwähnt habe, ist es ein Anti-HIV-Mittel. Deoxyuridin ist auch eine Strategie für die Herstellung von Derivaten, die einige dieser Infektionserreger in ihrer Wirkung stoppen. Eines dieses Moleküles, das auf der Basis von Desoxyuridin hergestellt wird, ist Idoxuridin. Es hat die gleiche Uracil Base wie Desoxyuridin, nur dass hier ein Jod an den Ring angehängt ist, wie man hier sehen kann. Diese Molekül wird zur Behandlung von Herpes simplex-Keratitis eingesetzt. Das letzte Molekül, über das ich hier sprechen werde, ist Trifluridin. Es hat eine ähnliche Struktur wie Uracil, aber in diesem Fall hat es ein 3-Fluorin Derivat, das ganz oben angehängt ist. Nun, all diese verschiedenen Moleküle, über die wir sprechen, sind in ihrer Struktur recht unterschiedlich. Sie haben eine gewisse Ähnlichkeit mit den Nukleosiden, die natürlich in den Nukleinsäuren vorkommen. Das Geheimnis der Art und Weise jeder dieser Funktionen ist, dass sie typischerweise einen gewissen selektiven Vorteil bei der Hemmung der Polymerasen von viralen oder bakteriellen Erreger haben, gegen die sie wirken sollen. Das bedeutet, dass die Polymerasen dieser verschiedenen Wirkstoffe, diese Wirkstoffe bevorzugt erkennen. Im Gegensatz zu den zelluläre Polymerasen, die sie eher ignorieren. Je mehr die Derivate also sein können wie die Polymerasen der Infektionserreger, desto wirksamer sind diese modifizierten Wirkstoffe und je weniger Nebenwirkungen werden sie haben. Wir haben eine Menge Zeit in dieser Vorlesung verbracht, um den Nukleotidstoffwechsel zu erklären. Wie die Purine hergestellt werden, wie die Pyrimidine hergestellt werden, wie die de novo-Pfade funktionieren, wie die Rückgewinnungspfade funktionieren, wie die Desoxyribonukleotide hergestellt werden, wie die Thymidin-Nukleotide hergestellt werden und dann schließlich zusammengefasst, wie unser Wissen über all diese verschiedenen Nukleotide zur Herstellung von Medikamenten genutzt wird, um Infektionserreger zu stoppen, die die menschliche Gesundheit beeinträchtigen.
About the Lecture
The lecture Analogs: Adenosine, Deoxycytidine, Guanosine/Deoxyguanosine, Thymidine, Deoxyuridine by Kevin Ahern, PhD is from the course Purine and Pyrimidine Metabolism.
Included Quiz Questions
Which of the following is not used in HIV treatment?
- Fleximer
- Zidovudine
- Abacavir
- Lamivudine
- Stavudine
Which of the following is not true regarding BCX-4430?
- BCX-4430 acts as an anti-translation agent and kills the infected host cells.
- BCX-4430 (Immucillin-A) is an anti-viral drug, an adenosine analog.
- The phosphorylated form of BCX-4430 acts as a competitive inhibitor of ATP.
- BCX-4430 halts RNA synthesis inside the cells.
- Immucillin-A acts as an anti-viral agent against the Ebola virus.
What is the mechanism of action for Zalcitabine (ddC)?
- A chain terminator during DNA synthesis
- A translation initiator during protein synthesis
- A transcription initiator during RNA synthesis
- A translation terminator during protein synthesis
- A transcription terminator during RNA synthesis
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Has high yield infos which are not in other lecturer's lectures
Brief, understandable, high yield information explained with illustrative slides by Dr. Ahern