Playlist
Show Playlist
Hide Playlist
Acid Base Balance and Anesthesia – Hemostasis and Acid Base Balance
-
03 - Hemostasis and acid Base Balance.pdf
-
Download Lecture Overview
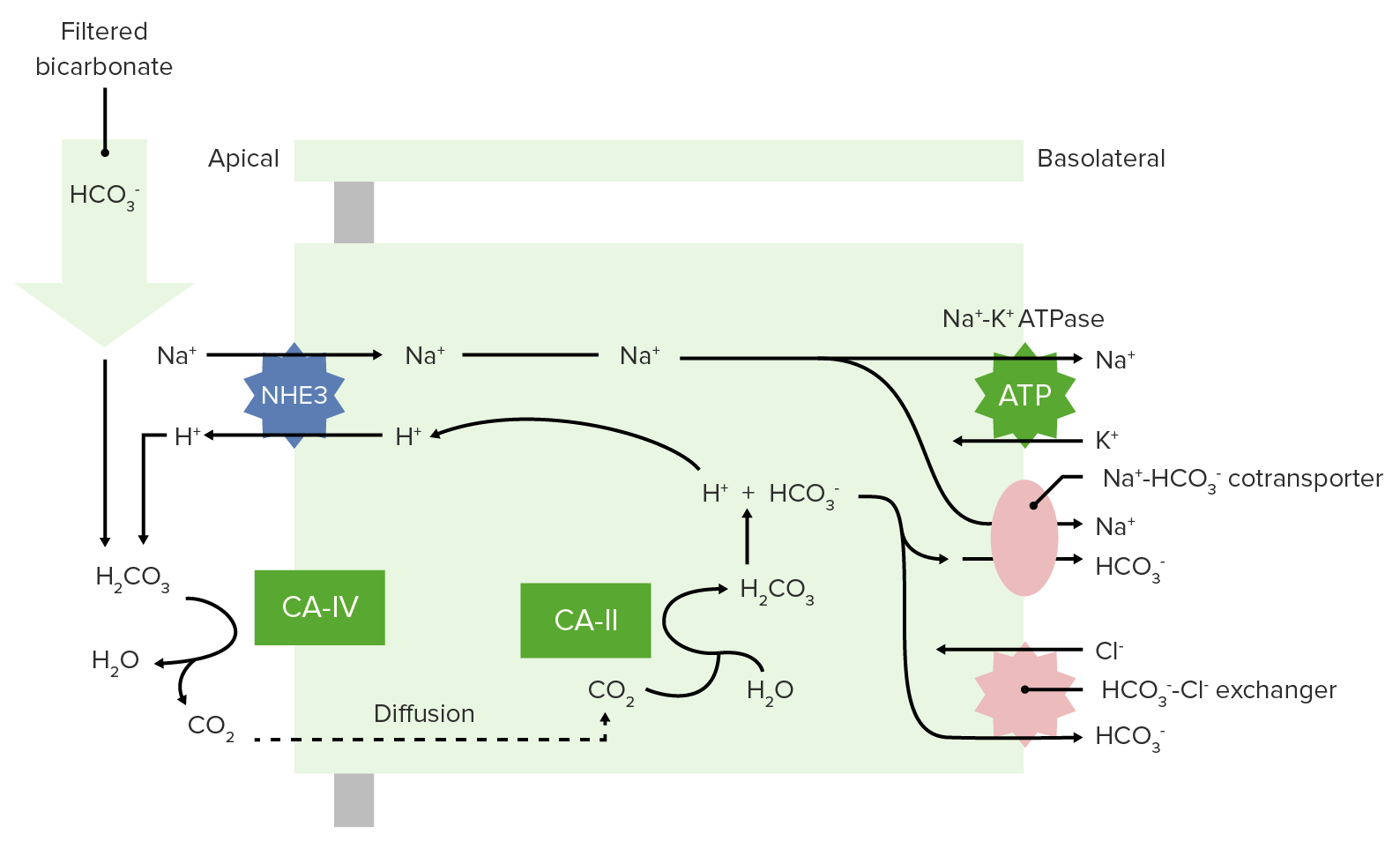
00:00 How about acid-base balance? Does general anesthesia have an impact upon acid-bases balance? The body's functions are only active through a very narrow range of pH, and it's the enzymes that only function properly between a pH of about 7.35 and 7.45. The closer you are to 7.4, the better. Neutral pH is 7, so the body is always slightly alkalotic to function optimally. If you ever let it drop down to 7, you're in real trouble. Anesthetic drugs do not affect the body's acid-base balance directly, but the actions of the anesthesiologist can have a profound effect on pH. In the section on Effects on Respiration, we've already mentioned that monitoring and control of ventilation can be an important part of the anesthesiologist's work. Over breathing causes a drop in blood carbon dioxide, which is known as respiratory alkalosis, which can have a negative effect upon many body functions. The opposite, letting CO2 rise, underventilating the patient can cause respiratory acidosis. 01:14 Mild respiratory acidosis, as I mentioned, is well tolerated, but if bicarbonate is reduced, in the presence of a metabolic acidosis, metabolic acidosis as opposed to respiratory acidosis, it may not be possible to control pH effectively by moderating the ventilatory function of the patient. In the section on Cardiovascular Function, where we've mentioned the importance of maintaining blood flow to vital organs, reduction of blood flow can cause reduction in oxygen delivery to tissues or hypoxia, which basically is suffocation of tissues. Hypoxia, or low oxygen in tissues, and hypoxemia, which is low oxygen in blood, both cause tissue acidosis, or metabolic acidosis, which has a major adverse effect upon body functions. 02:08 Hypovolemia, low total body blood volume as would happen with bleeding or dehydration, can also lead to decrease in oxygen delivery and blood to vital organs, which increases the probability of metabolic acidosis forming. Some diseases, particularly kidney disease, also have major metabolic effects. Because, in the presence of kidney disease, the ability to buffer acidosis with bicarbonate, which the kidney produces, is impaired.
About the Lecture
The lecture Acid Base Balance and Anesthesia – Hemostasis and Acid Base Balance by Brian Warriner, MD, FRCPC is from the course Anesthesiology: Introduction.
Included Quiz Questions
Which of the following statements about anesthesia and acid-base balance is FALSE?
- Anesthetic drugs themselves have a profound impact on the body’s pH.
- Overbreathing, resulting from hyperventilation, causes a drop in blood carbon dioxide.
- Under-ventilation can allow carbon dioxide to increase.
- Mild respiratory acidosis is usually well-tolerated.
- If bicarbonate is reduced in a patient with mild respiratory acidosis and metabolic acidosis is present, all body functions are adversely affected.
Which of the following statements about anesthesia and acid-base balance is FALSE?
- Hypoxia but not hypoxemia can cause tissue acidosis.
- Reduction in blood flow can cause hypoxia.
- Hypoxemia is defined as low oxygen delivery in the blood.
- Hypovolemia leads to metabolic acidosis through a reduction in blood supply to tissues.
- Kidney disease is particularly relevant to acid-base regulation and can cause metabolic acidosis through a loss in the production of buffers.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |