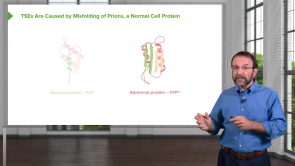
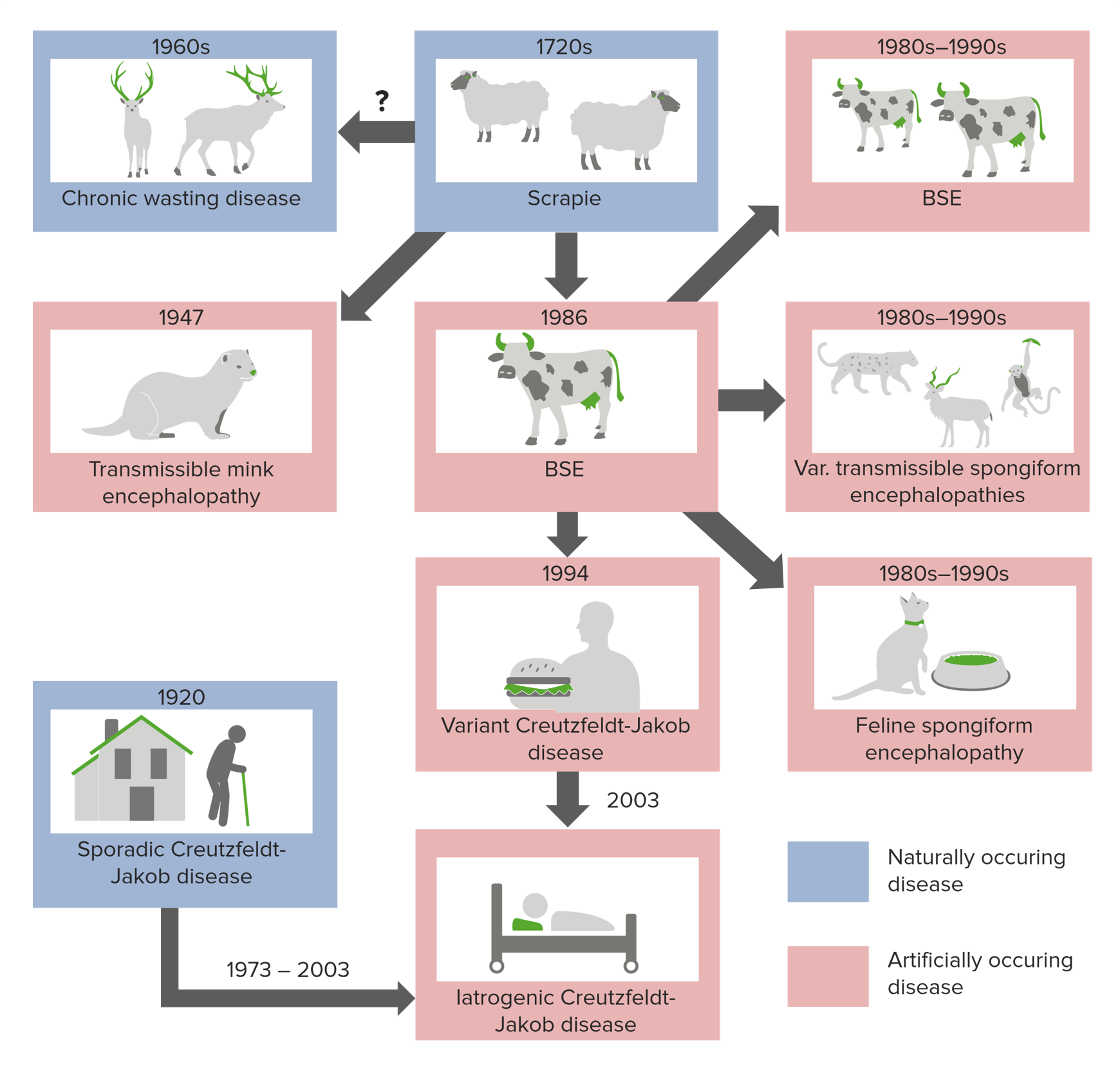
Really Gr8!
By Joel C. on 26. February 2023 for Molecular Test for TSE Prions – TSEs
really gr8. to the point. fascinating. this whole program is quality cme. _JC
My review
By Ahmed A. on 11. November 2020 for Prions
I haven't reviewed the lecture but personally from watching some of the previous vids I feel that doc Vincent is not giving a USMLE lecture. while what he does is very good, lecturio is very specific for USMLE, the lecturer has to hit the most high yield points in the most concise and easy to understand way.
the benchmark would be Hassan sattar in pathoma, very on the point summarizing massive topics to a single slide.
claro y conciso
By Cesar L. on 17. March 2020 for Transmissible Spongiform Encephalopathy (TSEs) of Humans
es un excelente vídeo, y esta muy bien explicado; justo el tema de mi próxima evaluación
I really liked it!
By Mariia R. on 04. June 2018 for Prions
By my opinion, very interesting and informative lectures. Thank you very much!
Prions
By Mark A. on 01. May 2018 for Prions
This guy is the best lecturer on this platform, knows the subject matter, knows how to capture your attention and also has a way of going over everything in a short summary
very informative and catchy
By Huriye Tuğçe A. on 27. November 2017 for Prions
I love his courses! Really interesting and informative! Thank you for this awesome lecture.
up to date information
By Sandra F. on 27. October 2017 for Prions
Very up to date information about prions. Professor Racaniello makes every lecture interesting.
Very nice
By Eunbi C. on 16. October 2017 for Prions
He is very passionate lecturer. His lecture is very engaging and interesting. Thanks for your lecture
Prions and statistics
By Rebecca S. on 22. February 2017 for Prions
This lectures goes through the important prion diseases. It focuses a lot on statistics making the lecture a littler longer that needed.
Overall a good lecture if you're not too busy in your studying
I like it for a general class, not for last minute revision.
By Bhavna P. on 04. February 2017 for Prions
It was very informational , I mean too much statistics about the history. Its really good for a class. But its bit TIME CONSUMING for some one running against time. One should be able to to hit guess the key points for revision.
Im sorry, but this is my honest opinion.