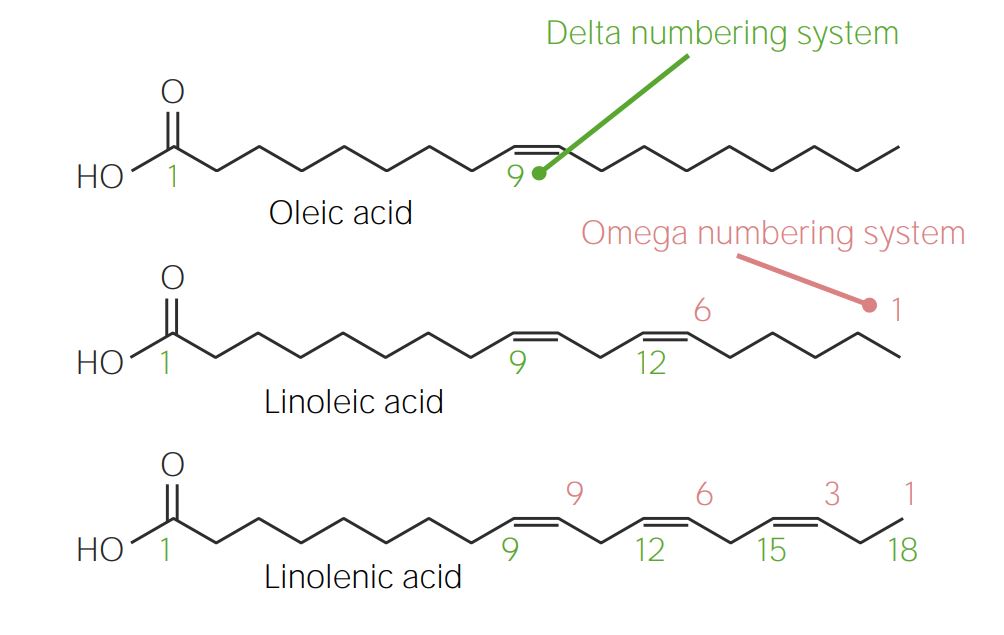
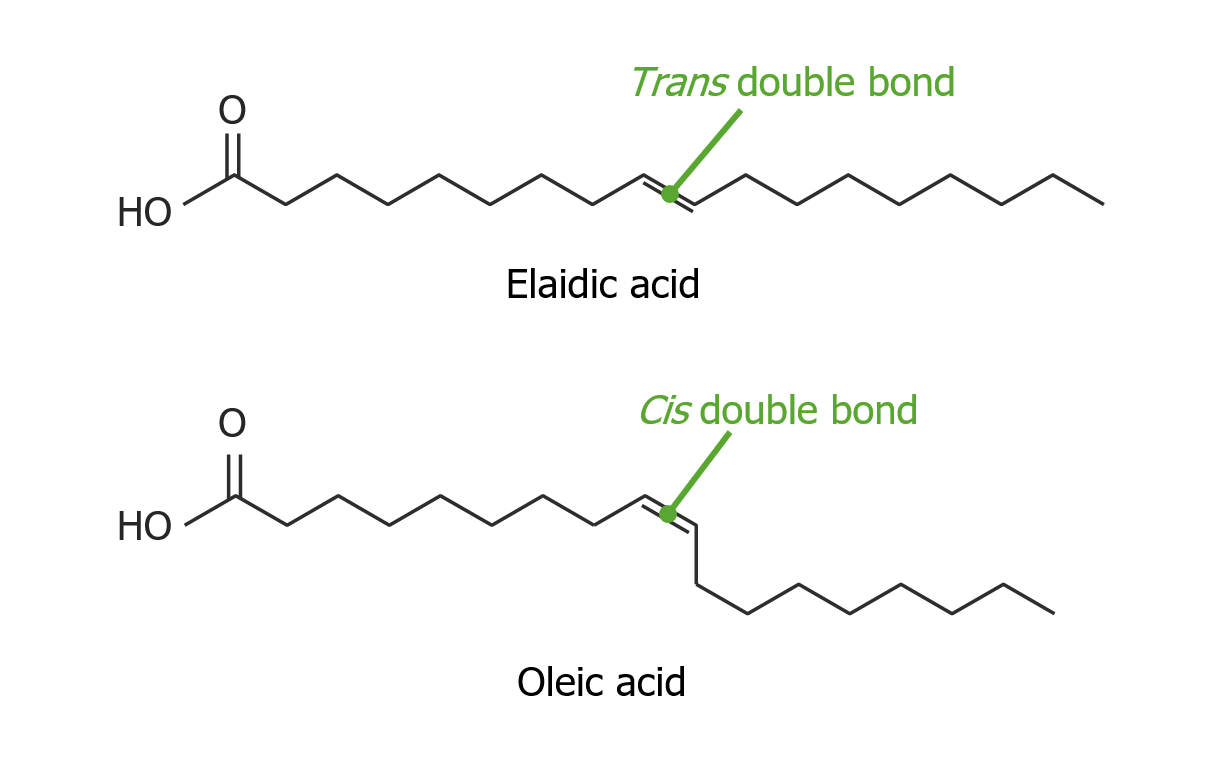
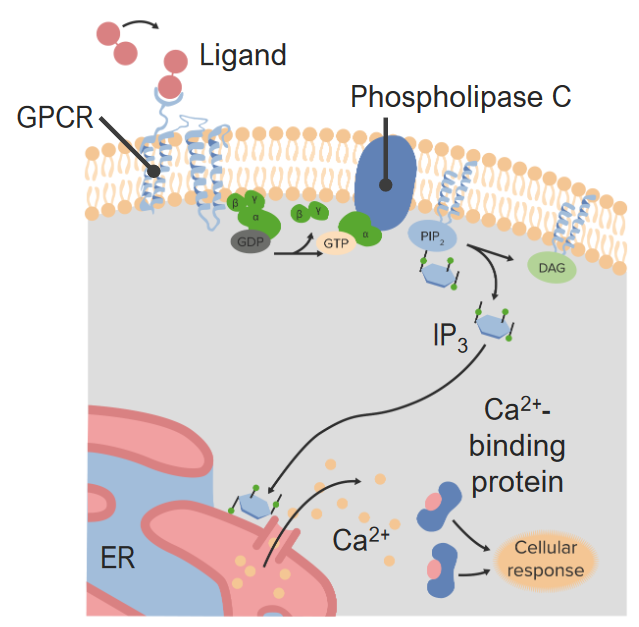
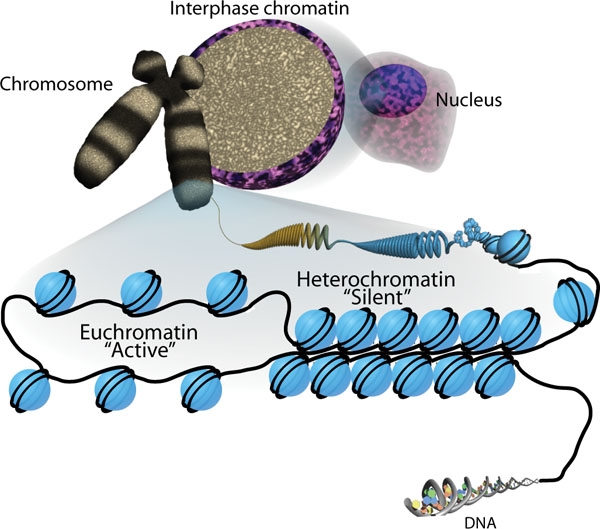
informative and intriguing
By Ali I. on 30. March 2024 for Ion Channel Blocker and Opener – Biological Membranes
informative and interesting way of conveying the subject which helps understanding them much easier.
Dr. Ahern
By Fadi J. on 22. November 2023 for Electron Carriers – Oxidation and Reduction in Metabolism
Dr. Ahern is amazing at linking what he's about to teach to previous videos.
Awesome course!
By Roberto E. on 01. November 2023 for Biochemistry: Basics
So far so good. The videos are easy to follow and I love the questions that pull up at the end of the videos to recall the information. Looking forward on finishing the rest of the modules in biochemistry, including all the other sections.
Errors in the Video
By Peddi A. on 12. October 2023 for Saccharides – Simple Carbohydrates
The following are the errors in the information you provided:
00:24 The general formula for monosaccharides is Cx(H2O)x, where x is equal to the number of carbon atoms in the monosaccharide. For example, the structural formula for glucose is C6H12O6, which means that it has six carbon atoms and six water molecules.
01:15 The structural formula for lactose is C12H22O11. This means that it is a disaccharide made up of two monosaccharides, glucose and galactose.
01:39 Polysaccharides are polymers of monosaccharides, but they do not necessarily have to have the same repeating sugar unit throughout. For example, amylopectin is a polysaccharide made up of glucose molecules, but it has a branched structure.
Here is a corrected version of the information you provided:
Carbohydrates are molecules whose name literally means hydrates of carbon. This is because monosaccharides, the simplest carbohydrates, have a ratio of one carbon atom to two hydrogen atoms to one oxygen atom, the same as water.
The general formula for monosaccharides is Cx(H2O)x, where x is equal to the number of carbon atoms in the monosaccharide. For example, the structural formula for glucose is C6H12O6, which means that it has six carbon atoms and six water molecules.
Disaccharides are carbohydrates that are made up of two monosaccharides. They are formed by a glycosidic linkage between the two monosaccharides. Disaccharides have the general formula C12H22O11. Examples of disaccharides include sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (glucose + glucose).
Polysaccharides are carbohydrates that are made up of many monosaccharides. They are formed by glycosidic linkages between the monosaccharides. Polysaccharides have the general formula (C6H10O5)n, where n is the number of monosaccharides in the polysaccharide. Examples of polysaccharides include cellulose, starch, glycogen, and chitin.
Polysaccharides do not necessarily have to have the same repeating sugar unit throughout. For example, amylopectin is a polysaccharide made up of glucose molecules, but it has a branched structure.
Needs more problem solving, and new quizz.
By vicky s. on 03. October 2023 for Biochemistry: Basics
The lectures are not bad, they have the material, tbey are very well spoken and easy to undersgand. I find some quizz questions to not be relevant, i believe i won't be aske dthese questions on exam. And sometime the material itself feels dry. I am more of a doers, so by solving more complicated questions with the help of a professor would my learning experience much better. But over all not a bad course.
Good signs of a lecture.
By Kausar F. on 17. August 2023 for R-group Categories – Amino Acids
Simple, not over-complicated and understandable, all great signs of an amazing lecture.
excellent
By Maymoona W. on 29. April 2023 for Lipid Links – Complex Carbohydrates
it's very easy and simply explained and I loved it
It was very helpful
Review
By Austin B. on 28. March 2023 for Involved Proteins – Protein Movement and Cell Signaling
I am appreciative that Dr. Ahern took the time to update his lectures. Shows he cares. Thank you!
One of the basic and topest of medicines headlines.
By Foday T. on 21. January 2023 for Biochemistry: Basics
Bio means life, chemistry means the composition of matter and energy requirements for life to exist. Chemicals and anyone
God bless lecturio
By Oluwadamilare A. on 08. December 2022 for Biochemistry: Basics
It was just what I really needed
I love lecturio so much
Gratitude
By Patricia T. on 03. November 2022 for Gene Expression – Metabolism and Regulation
All the lectures have been so, so helpful. Thank you so much!
Biochemistry like live
By Musab AbdAsamed A. on 08. October 2022 for Biochemistry: Basics
Kevin is Amazing man, his body language get a lot to his explanation!
excellent
By Carlos C. on 09. September 2022 for Biochemistry: Basics
excellent explanation of topic very difficult to understand for a med student
Perhaps some mneumonic would help
By Babatunde A. on 09. May 2022 for Biochemistry: Basics
Nice delivery but as much as Dr tries to break it down,it looks a bit abstract.
Perhaps some mneumonic would help
Easy to understand and memorize
By deleted u. on 06. April 2022 for Biochemistry: Basics
Professor Kevin make it enjoyable course; It helped not only in remembering many info that I forgot in the class, but also I did learn many new valuable information. The fact that the explanation provided by Dr. Kevin is so easy to understand and memorize, make this course very interesting.
Biochemistry on lecturio is superb
By Chigozie A. on 06. April 2022 for Biochemistry: Basics
I am a biochemistry student, the videos on lecturio have been so amazing. The lectures are superb. The language is easily understood and in a very friendly way. The concepts are been discussed fully and appropriately. It has made me to understand biochemistry more.
ok
By Liviu I. on 24. March 2022 for Biochemistry: Basics
ok ok ok ok ok ok ok ok ok ok ok ok
actin
By ??? ???? ????????? ا. on 02. March 2022 for Globular Structural Proteins – Protein Movement and Cell Signaling
just got the structure formation of a f actin support epithelial
Great discussion!
By Kharieza C. on 19. February 2022 for Biochemistry: Basics
I like how Dr. Kevin discussed all the topics, simple and easy to understand.
Knowing the fundamentals
By Leslie Gabriela S. on 22. January 2022 for Involved Proteins – Protein Movement and Cell Signaling
The lecture breaks it down so simple that it’s easier to understand. There’s still more factors that I’m sure they’ll be discussed in other lectures but it’s perfect to help to create a strong foundation in your knowledge
Loved it
By Mishal W. on 12. January 2022 for Biochemistry: Basics
Great Visualization and the elaborative method of teaching is right according to my mind.
clear speak, high yield
By Jamshed B. on 04. January 2022 for Biochemistry: Basics
brief , clear, high yield, relaxed, in depth lectures. With a question and answer section at the end of every lecture. I'm loving it
Biochemistry
By Musiime F. on 14. December 2021 for Biochemistry: Basics
Dr Kevin explains the concepts slowly and with emphasis. He also includes the physiological aspects, making biochemistry sound more biological rather than chemical
5
By Robinson P. on 13. December 2021 for Ionization – Amino Acids
I loved this lecture ´cause dr ahern was so clear and it really helped me understand this topic.
Helpful
By Iman a. on 04. December 2021 for Biochemistry: Basics
The professor teaches very well, and difficult concepts are taught in a clear, concise manner.
Biochemistry
By Rabail R. on 03. December 2021 for Biochemistry: Basics
Basics of this course can be explained in a very detailed manner
Biochemical molecules
By Ahmad A.majid H. on 15. November 2021 for Biochemistry: Basics
The lecture was simple understandable and Clear , it gives a broad explanation about the fundamentals of biochemistry
Made something so complex easy
By Prashant U. on 29. October 2021 for Catabolism and Anabolism — Metabolism and Regulation
Made something so complex easy. I've always had trouble here.
Explained well
By Prashant U. on 07. October 2021 for Structure – Amino Acids
I loved this lecture! Everything was so well explained: simple, straightforward, and I especially love few external tidbits thrown in such as the meteorite portion. It helps to remember these facts. Thank you!
Finally it makes sense!
By Prashant U. on 07. October 2021 for Hydrophobic R-groups – Amino Acids
This lecture was one of my favorites as it made something so confusing perfectly digestible.
GOOD
By Tasneem B. on 07. October 2021 for Bilayer Composition – Biological Membranes
Dr. Ahern is a very good lecturer. He explains concepts very clearly.
Thank you.
By deleted u. on 29. September 2021 for Biochemistry: Basics
Excellent resource. Thank you. I also enjoy the quizzes and practice medical scenarios.
Clear and short
By Gabriela Sofia O. on 20. September 2021 for Sodium-potassium ATPase – Protein Functions
He explains on a perfect and short way making it easier to understand
Grandma getting her Medical degree in Neurosurgery!
By Christina G. on 27. August 2021 for Biochemistry: Basics
Excellent review of the basics; been a practicing Doctor of Chiropractic for 27 years and your videos are bringing it all back again! On the way to taking the MCAT to get my medical doctorate in Pediatric Neurology specializing in neurosurgery.
Planning on beginning practice when I am around 68/69...my second career. Thank you for helping me do this great endeavor!!
A life changing choice!
By Daira Nerea M. on 06. August 2021 for Biochemistry: Basics
Five-star score not only for the high-yield information but also for the excellent teachers, spaced repetition tests and articles.
Thank you all for bringing this amazing learning format!!
Perfecto!!
By halima u. on 26. July 2021 for Biochemistry: Basics
Really good explanation. And I like the professor. I understood
Add more clinical correlation and bridge to other areas
By Herbert G. on 13. July 2021 for Biochemistry: Basics
Please is important to add more clinical correlation, clinical cases and bridge to other areas: pharmacology, microbiology, etc.
Usmle test the integration of knowledge.
Thrist quencher
By eyob a. on 28. June 2021 for Biochemistry: Basics
Interesting.
Mental thirst quenchinly good.
Interconnected. Just enough info.
Dr.kevin fun lectures.
ok
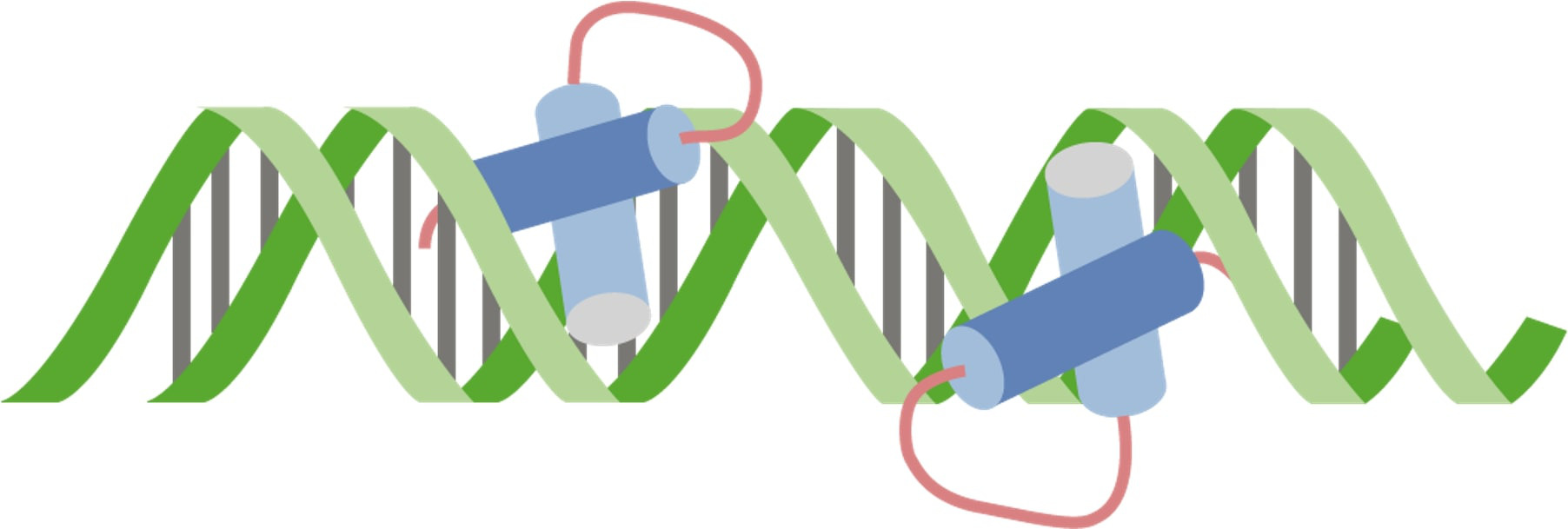
By eyob a. on 26. June 2021 for Involved Proteins – Protein Movement and Cell Signaling
ok. could use improvement
please include that Kinesin and Dynein are ATPases that use energy.
also that only Kinesin is displayed here. I was so busy looking for Dynein information, that Kinesin info went out the other ear. This could be better by improving the diagram.
Excellent presentation of Biochemistry
By Clarissa N. on 17. June 2021 for Biochemistry: Basics
This course is an excellent presentation of biochemistry. Professor Ahern presents the material in a clear, concise, easy to understand format that is easy to follow. I really like that I can pick and choose the lectures I need to watch, particularly those that I need to prepare for my upcoming exam. I find the review questions very helpful. Overall, I am finding the course very helpful and I am looking forward to completing more of it.
Great class
By Elizabeth Y. on 01. June 2021 for Biochemistry: Basics
I love it! It's clear and concise! Dr.Ahern is a gem to learn from. He breaks it down to where I can understand.
Love it!
By Aderonke I. on 31. May 2021 for Biochemistry: Basics
Nice speaker and illustrations. The lectures were engaging. Thank you!
Good lectures!
By Mathilde N. on 07. May 2021 for Biochemistry: Basics
Kevin Ahern is a really good teacher, I really enjoy his lectures, but sometimes it's hard to follow the lecture due to the presentation picturing one thing and him talking about something else. And also when he's talking about about the presentation I wished he had some sort of pointer so I would be easier to know what structure he's talking about????????
perfect
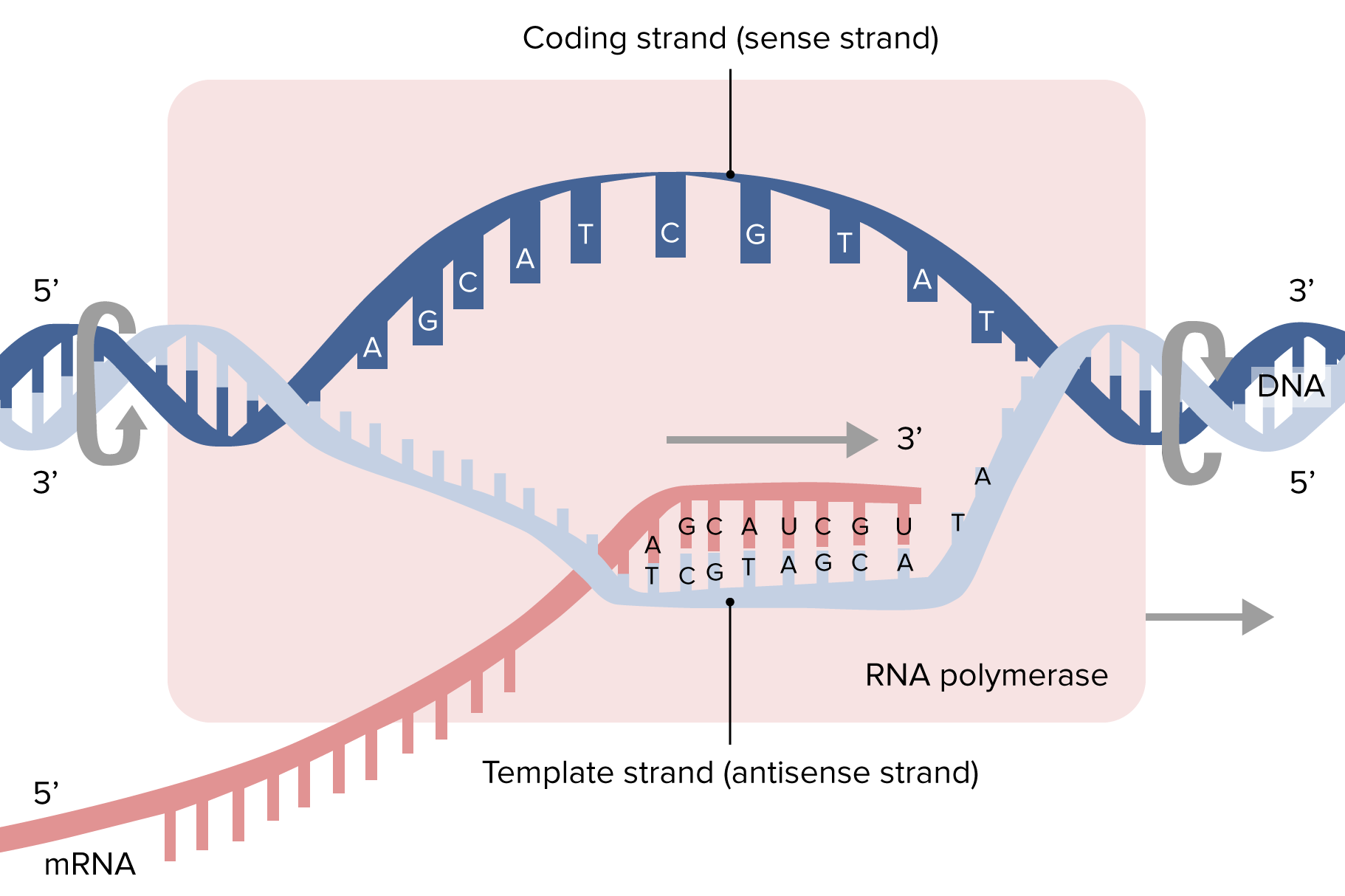
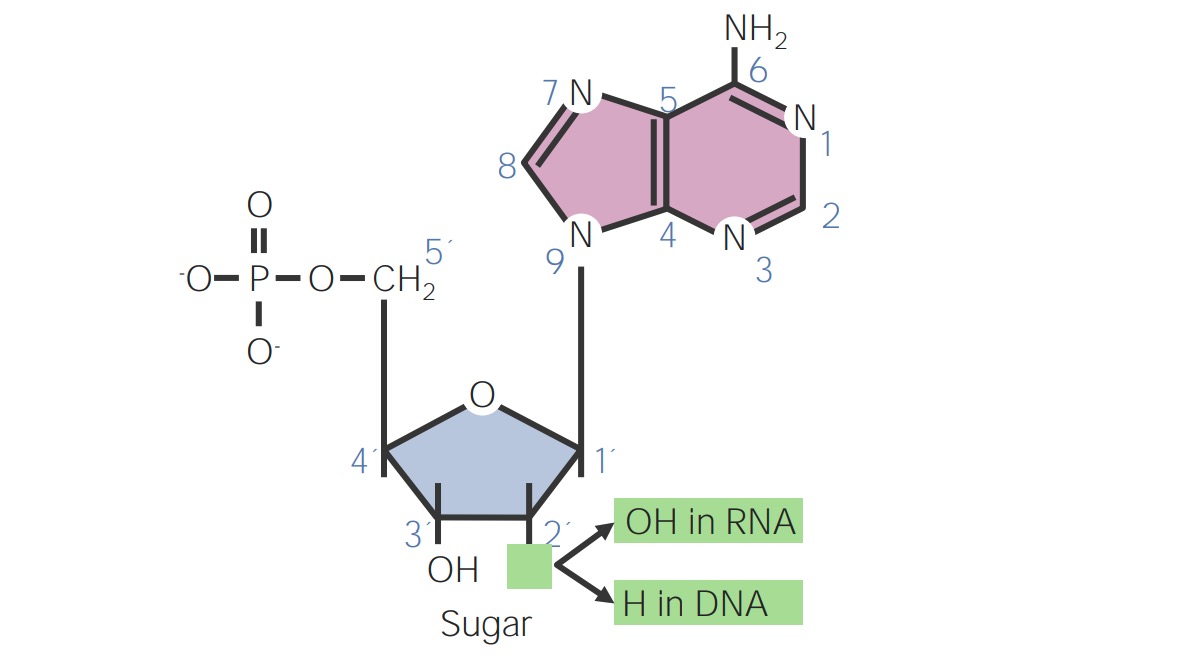
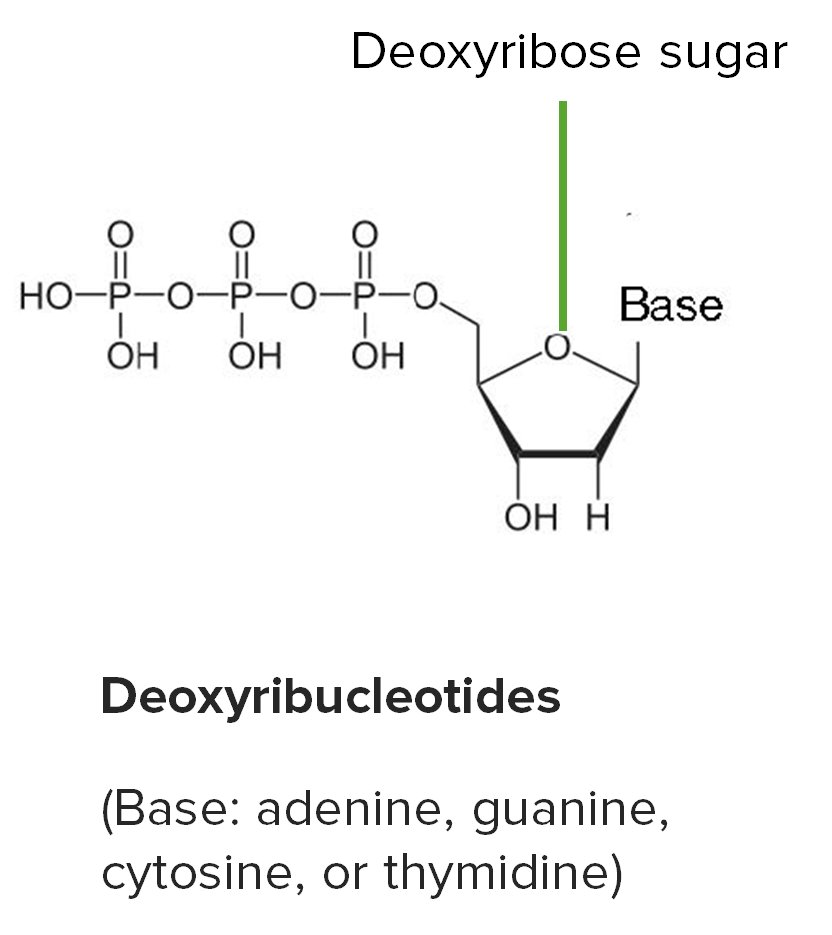
By shalu b. on 25. April 2021 for Nucleotides – DNA, RNA and the Genetic Code
Beautiful and very good explanation . easy slides. thankyou. study matter also provided
Excellent services
By Mohammad J. on 10. April 2021 for Biochemistry: Basics
The presentation is very nice and helpfuly
The organization of topics was very good
High yield information.
By deleted u. on 01. April 2021 for Biochemistry: Basics
I'm impressed with how high yield the information is. The professor touches very important aspects in just the shortest time that one video has, and that is really impressive. I do have a degree in Biochemistry and molecular biology and so this is really making me master the course gradually. For sure I'm recommending this course to my friends from same college already who aspire studying medicine like me.
Really good topics
By Carlos A. on 08. March 2021 for Biochemistry: Basics
The videos are shorts and specific an also great explanations
Wonderful lectures
By Melvin K. on 06. March 2021 for Biochemistry: Basics
Great explanation with great examples . Knowledgeable in subject matter , content up to date
TRADUCTION
By EDUARDO R. on 04. March 2021 for Gene Expression – Protein Functions
THE TRANSLATION IS NOT CORRECT, THERE IS NO CONSISTENCY IN THE SENTENCES
'Biochemistry Basics' a wonderful presentation of the basics of Biochem!!
By Ian E. on 28. February 2021 for Biochemistry: Basics
Thankyou so much for the opportunity to relearn most of the important and key concepts related to this cornerstone foundation to Medicine & Life Sciences! Wish I had this tool in my undergrad days!!
????????????
By Neuer N. on 21. February 2021 for Communication and Signaling – Protein Movement and Cell Signaling
He is the best here , I swear
Idk but I like him
Lecturio's Biochemistry
By Hikmet G. on 18. February 2021 for Biochemistry: Basics
Great course for introductory level biochemistry. Prof. Kevin Ahen is a great teacher, knowing not only the topic but it's historical background...
Hmm I like him
By Neuer N. on 12. February 2021 for Biochemistry: Basics
He's a legend ooh I don't know what else should I say to complete 10 words
biochemistry tough as havent chemistry before. Had to keep reviewing, loved the tests and course presentation.
By robin s. on 11. February 2021 for Biochemistry: Basics
biochemistry was tough as I havent chemistry before. Had to keep reviewing, loved the tests and course presentation and so satisfying when i got the answers right. Great system for people like me . cant wait to do other subjects when i finish this one.
best professor ever
By vivek k. on 31. January 2021 for History – Introduction to Biochemistry
what an amazing lecture
god bless u sir and i wish to meet u someday
Thank you so much & Feedback (you will have the answer to the problem)
By Shang Ya C. on 24. January 2021 for Gene Expression – Metabolism and Regulation
Thank you so much; Nonetheless, i really need to let you know your vocabulary is above my reach
Precise and accurate
By Melchora F. on 19. January 2021 for Secondary Structure – Peptides
I understand very well, thank you Professor Kevin, these help me a lot
Great biochem review.
By Yuriy C. on 14. January 2021 for Biochemistry: Basics
It was much needed review for me in general. Thank you.
Great Job!
By Joseph G. on 14. January 2021 for Biochemistry: Basics
Enjoyed these videos very much. They helped me understand concepts I was struggling with.
Clear and understandable
By Brent H. on 14. January 2021 for Biochemistry: Basics
Great work here,,, I’m so happy for the clearly understandable English
More time necessary for proper grasp of the Information
By OKWIR A. on 07. December 2020 for Biochemistry: Basics
I just find the need for an extra time revising this, particularly offline.
I always find my self making some notes unlike Anatomy!
Too fast, not details
By Rasool G. on 28. November 2020 for Biochemistry: Basics
I think it’s too fast, and not with details so please correct these problems
Great
By Ian P. on 28. November 2020 for Polysaccharides – Complex Carbohydrates
Magnificent lecture about polysaccharides and their functions for storage and release
Precise
By Ian P. on 24. November 2020 for Hydrophobic R-groups – Amino Acids
The video highlights the important concepts about the hydrophobic amino acids group
Good not too much good just happy
By Rashid k. on 28. October 2020 for Biochemistry: Basics
It's is just good that's why 4. I dislike that it's doesn't explain clinical too much
A Easy way of teaching
By Otaru O. on 26. October 2020 for Biochemistry: Basics
It's okay.... But somehow hard to comprehend. I will love if you can make the teaching much easier to grasp.
great
By Mai L. on 10. September 2020 for Structure – Amino Acids
this video is short and easy to understand. Thanks Teacher
Translation
By Joseph A. on 08. September 2020 for Translation – DNA, RNA and the Genetic Code
Good explanation. Pretty bad diagram. Animation probably would work better.
Clases de bioquimica
By Maria jose P. on 02. September 2020 for Biochemistry: Basics
Me encantan las clases son muy explicativas y muy detalladas , simplemente excelente!!!
this video helped me so much and i improved my grades
By Neuer j. on 31. August 2020 for History – Introduction to Biochemistry
i enjoyed every second of this video and it opened my mind to study more and become what i have always wanted to be a radiologist
Essential lecture materials
By Lisa R. on 25. August 2020 for Common Sugars: Nomenclature & Structure – Simple Carbohydrates
Concise and clear; exactly what I needed for this topic, thank you.
Excellent
By Olive O. on 24. August 2020 for Biochemistry: Basics
Good amount of detail in digestable amounts. Very much appreciate able to run the videos at different speeds.
boring .. like reading a book
By Mustafa N. on 23. August 2020 for Structure – Amino Acids
No explanation at all just reading , sorry for that , No interaction
Just Amazing!
By Patrick Lance S. on 17. August 2020 for Biochemistry: Basics
It's great to learn from someone who's enthuisiastic about their specialty and even better at teaching it. Thank you Dr. Ahern (:
This review is very good
By Tina J. on 23. July 2020 for Biochemistry: Basics
I like the short videos. They described what’s important and later you get questions about the video.
Fantastic class material but the quiz questions need improvement
By Mia H. on 26. June 2020 for Biochemistry: Basics
I love having access to such rich learning material, thanks for making this an available resource. My only criticism would be that the quiz questions could use improvement and the professor could talk slower to make note taking easier. Overall, very grateful to have found this!
Useful
By SABRINA Y. on 12. June 2020 for Biochemistry: Basics
Understandable!!! Easy to remember! And pretty useful!!! You really need to learn basics topics in Med career it surely make you to be highlighted over another students
Really Good
By BRENDA L C. on 10. June 2020 for Biochemistry: Basics
It helped me clarify some topics I had been having trouble with.
My mistake
By Luis David U. on 08. June 2020 for R-group Categories – Amino Acids
Sorry, the following videos have the information that I was looking for, sorry again
GREAT :D
By Vale A. on 15. May 2020 for History – Introduction to Biochemistry
I loved it, really interesting and easy to comprehend, thank you very much :)
Good
By Aipathi rao u. on 12. May 2020 for Biochemistry: Basics
Need more mcq 's for practice and good content. Very clear concept s
Thanks
By Binisha B. on 08. May 2020 for Biochemistry: Basics
Very good
Thanks
Really it doesn't take much time and its really good
the presentation is very comprehensive
By kofi o. on 06. May 2020 for Biochemistry: Basics
the lecrure is detailed and very comprehensive
it is well presented
i also liked the quiz that comes afer the lecure.
could be improved
By Cherry E. on 30. April 2020 for R-group Categories – Amino Acids
It almost always fails to explain 'why'. These lists are available in any textbook. I was looking for an explanation to understand it. Also, visually it is looking at a table for 5 minutes, pictures could have explained better.
Videos are well summarized ans full of usefull information
By Hategekamungu A. on 26. April 2020 for Biochemistry: Basics
Dear sir/madam
In regard to the Biochemistry basics,I am much satisfied by the cours.In fact,the videos concerning biochemitry basics are well summarized and are full of useful information in the regard of médical studies.Because of this,I am enjoying the course and continuing either to learn much more .
Yours sincerely
Asher Hategekamungu
Awsome course
By Jose Arturo G. on 19. April 2020 for Biochemistry: Basics
Dr. Ahern is a fantastic lecturer. All the course is simply awsome.
Easy to follow course
By Brandon A. on 12. April 2020 for Biochemistry: Basics
The course was easy to follow and has good illustrations of the concepts. I also like the quiz portion of the course.
Has really good biochemistry summaries.
By André M. on 05. April 2020 for History – Introduction to Biochemistry
Great professor and easy biochemistry topics to learn with good explanation.
Learning biochemistry
By Greg B. on 01. April 2020 for Biochemistry: Basics
I work in a large public hospital biochemistry routine department, when I studied this subject there where no computers, only text books. So this is a refresher and update of the subject. I am enjoying this 'new' way of learning and it enhances my work knowledge as understanding theory is important for performing work well.
Amazing!
By Abdullah F. on 01. March 2020 for Biochemistry: Basics
I like his style and respect his effort. I've been using these lecturers to review my knowledge
Preparation for step1 and step2 of ECFMG examination
By andre g. on 27. January 2020 for Biochemistry: Basics
hi
Biochemistry's videos are interesting and give me information, the orator pronounce correctly.
As you know I do my best to prepare ecfmg exam and board examination next, of course after having sufficiently training and advices under your guidance
I would like to apply ecfmg examination this year, now would you please give me more information for preparing step one and step 2 exam.
I
By Mary S. on 15. December 2019 for Structure – Amino Acids
two stars just for the effort. But its ore like reading fro book. Nothing different. Very boring. No interaction.
Simple,informative and explains very well
By Patwant S. on 29. November 2019 for Oxygen Transport – Protein Functions
Talks clearly and explains all the key points so that you can understand the lecture in simple terms
I am trying to studying ABC of every single subject
By Lina Q. on 13. November 2019 for Biochemistry: Basics
Studying Biochemistry lectures helps me to understand and hold in memory every each case,,, so this is the best ever
Biochem
By Ekene O. on 24. October 2019 for Biochemistry: Basics
It’s not a bad lecture but it’s bulky stuff that isn’t simplified to hold ones attention, don’t get me wrong, it has its highlights but it could be more interesting.
I like it to the core
By Bhavya S. on 24. September 2019 for Biochemistry: Basics
All the explanation are precise to the point...and covers all the important concepts
Awesome Biochem lectures
By Hailey S. on 12. September 2019 for Biochemistry: Basics
The professor is excellent. I like how he explains things. Some of the professors move their arms around a lot which can be distracting or spend the whole time looking down at the computer. With others it feels like all the do is read from the slides. However the biochem lectures are very well done and by far my favourites so far.
I love it!
By Eli O. on 30. July 2019 for Overview – Introduction to Biochemistry
Thank you for your knowledge and for spreading it in this way for us, the students.
Fascinating work of nature
By Kabwea T. on 19. July 2019 for Biochemistry: Basics
The lecturer makes the topic interesting, easy to understand.
One begins to appreciate how these things link with everyday human existence and how they play a pivotal role.
nice lecture!
By Rong F. on 28. June 2019 for Other Lipids and Their Function – Lipids
its good and thorough, also covers minor lipid types! good to learn
Excellent
By Daniela B. on 15. June 2019 for TRNA Charging and Ribosomes – DNA, RNA and the Genetic Code
Explains it completely from the start to the end , everything is clear and simple . The structure is very easy to understand.
Very helpful
By Joaquin Vazquez J. on 11. May 2019 for Overview – Introduction to Biochemistry
I am a student at Downey High and I really want to be a cardiothoracic surgeon, and I didn't know what to learn but I know this website will help me.
biochemistry at basic
By dr. Jadumoni g. on 24. April 2019 for Biochemistry: Basics
just basic to litle details course.easy to understand it.
Not boring !
By Geraldine C. on 20. April 2019 for History – Introduction to Biochemistry
I think it is an entertaining way to learn the history of Biochemistry.
Amazing
By Frank V. on 10. April 2019 for Overview – Introduction to Biochemistry
I love this guy. The way he explains makes Biochem look easier
Short, simple, and clear
By Ofir Perez D. on 02. April 2019 for Oxygen Transport – Protein Functions
He is clear, short, and simplify the most important points
the first steps
By Ignacio R. on 27. March 2019 for Overview – Introduction to Biochemistry
he is very clear and is a nice teacher, and he use a appropiated nivel of technicims
like it but it seems that the PhD is so fast but I used the subtitles.
By Hussein A. on 13. March 2019 for Translation – DNA, RNA and the Genetic Code
I really liked this site ,and it's seems helpful for me .
I am a high school student. I am in the 10th grade right now. I just want to become a surgeon doctor because I want to save peoples lives. I know that I have to right a commit to this site and I do not have to introduce myself for you people. I do not need to study this because I did not understand today's subject.I just want to be ready for the medical school. I hope this website will help me being ready and ,Good Luck.
concise and to the point with relevant groupings of amino acids
By Vinh N. on 01. March 2019 for Hydrophilic and Ionic R-groups – Amino Acids
extremely helpful when the amino acids are divided in functional relevance.
It was helpful. Thank you.
By 92 0. on 24. January 2019 for R-group Categories – Amino Acids
:)
Thank you .
everything in this lecture is clear.
like it very much.
Yearning for more
By Ivan M. on 08. January 2019 for Overview – Introduction to Biochemistry
This is only the first lecture and the first time I am delving into biochemistry for the first time, so hard to say, but something about Prof. Ahern's manner makes me curious and creates a fine feeling of anticipation. Great for a first lecture.
great job
By Abida A. on 04. January 2019 for Gene Expression – Metabolism and Regulation
it was described in a simple and easy way to understand, thank you.
Where are the acidic amino acids?
By Sergio N. on 03. January 2019 for Hydrophilic and Ionic R-groups – Amino Acids
This video did not really show Aspartic Acid or Glutamic Acid?
He amazingly simplifies these things
By ari t. on 01. January 2019 for Structure – Amino Acids
He very easily simplifies things. So this is why I give him a five star review
Recommend it!
By Tiffany H. on 28. December 2018 for Overview – Introduction to Biochemistry
Amazing, loved it very much. Easy to follow and clear.
High yield, key points
By Alejandro Gonzalo V. on 12. December 2018 for Hydrophobic R-groups – Amino Acids
Awesome lecture, awesome proffesor. My teacher spent almost 1 hour explaining that. Thank you Dr. Ahern
could be better
By Dr AGNEL X. on 06. December 2018 for Biochemistry: Basics
as a beginer i didnt couldn't understand anything the lecturer is talking about and the lecturer is way too fast
Congrats!
By Barbara Alejandra C. on 04. December 2018 for Secondary Structure – Peptides
My name is Barbara, I'm from Colombia and I really like your videos Mr. Kevin, because I think the content is easy to understand.
Great lecture!
By Armin S. on 17. November 2018 for Polypeptides – Peptides
Very well explained and detailed lecture, but it would be nice for better understanding to have some names and terms (e.g. Ramachandran plot) displayed in the video!
Too fast
By Ida A. on 15. November 2018 for Hydrophobic R-groups – Amino Acids
way too fast ????I really wish the teacher would talk slower as now I keep having to pause and rewind etc.
Easy to follow
By Janet G. on 31. October 2018 for Overview – Introduction to Biochemistry
Direct , and easy to follow. The length of the video also helps.
Not necessary
By Claire U. on 17. October 2018 for History – Introduction to Biochemistry
Great professor but unfortunately the details of this lecture are not important for USMLE Step 1
Great
By Andreia S. on 15. October 2018 for Structure – Amino Acids
Easy to understand and the explanation are very clear. Thank you very much good job!
Details and Pace
By Abdulmohsen A. on 26. September 2018 for R-group Categories – Amino Acids
Needs more details, and take it slower so that we the audience can get a more thorough understanding of the topic (the video really doesnt need to be that short)
Good lecture
By Gabriela G. on 25. September 2018 for Structure – Amino Acids
I found it really helpful. Your explanations were clear and easy to understand.
Adam William Mason Doran
By Adam William Mason D. on 22. September 2018 for Structure – Amino Acids
Thank you for the video's theses are really helpful. look forward to keep learning.
well done lecture
By wafaa s. on 14. September 2018 for History – Introduction to Biochemistry
very nice clear and organized lecture. thank you. it adds alot to my simple knowledge
enriching lecture
By Badr L. on 11. September 2018 for Quaternary Structure and Summary – Peptides
thanks for teaching me in such a clear, precise, and yet enriching way
simply the best!
By Badr L. on 31. August 2018 for History – Introduction to Biochemistry
excellent lecture. Cristal clear; not too slow not too fast, smooth transitions and a bright ending
Precise Presentation.
By Antru S. on 20. August 2018 for Overview – Introduction to Biochemistry
It was very precise and up to the point. The presentation was interesting that triggers to study better .
exellent explanations
By roberto m. on 18. August 2018 for Ion Channel Blocker and Opener – Biological Membranes
very good lecture. pretty well organized and instructive. He has a deep knowledge of the material making me to pay special attention to all his lectures.
Very Good
By Arielle Karina T. on 16. August 2018 for Overview and Introduction on Fatty Acids – Lipids
Very nice and informative lecture, easy to understand and remember
Once again, simple and perfect explanation of Lipids.
By Suhas M. on 16. August 2018 for Glycerolipids and Glycerophospholipids – Lipids
I have followed Dr Ahern through all the basics of biochem lectures and he has never failed to deliver.
Thank you!
By Nathan A L. on 16. August 2018 for Biochemistry: Basics
Great job, Dr. Ahern. Thank you for bettering my understanding of these concepts! Used as a supplement to lectures and powerpoints, your videos on Lecturio have been extremely helpful to me!
Structure-Amino acids
By Sergei R. on 11. August 2018 for Structure – Amino Acids
Very good lecture, l consider that is one of the most complete themes that l´ve read ...good job.
Excellent information and a very useful method of biochemistry study
By Jose S. on 02. June 2018 for Biochemistry: Basics
Its a little bit dificult to understand but the information its complete and tables and graphics helps a lot
My doubts got cleared
By Maryam J. on 29. May 2018 for Transcription – DNA, RNA and the Genetic Code
Conveyed the process in a short &digestable way.I LIKED THE WAY OF presentation too.
"wow"
By mithra p. on 26. May 2018 for Overview – Introduction to Biochemistry
his teaching is clear and it is not allowing me to get even a single doubt
concepts understood!
By yamuna m. on 25. May 2018 for Biochemistry: Basics
i could understand the concepts better. explanation was good.the charts were self explanatory. it made me feel bio chem much more interesting.
Your explanation is making sense and connect biochemistry with real life!
By mei huah c. on 16. May 2018 for Communication and Signaling – Protein Movement and Cell Signaling
I like the way you link things from one to another. Yuu made these lectures highly appreciated with the concise and comprehensive explanation! Thanks Prof!
"wow"
By mithra p. on 30. April 2018 for Structure – Amino Acids
i like your teaching .sir. it helps me to understand the concepts clearly.
Thanks
By Gerardo C. on 30. April 2018 for Structure of Proteins – Protein Movement and Cell Signaling
Excellent information and explanation, it's helping me a lot. Congrats, lecturio!
Learn efficiently and broadly!
By Yen Chun L. on 03. April 2018 for Biochemistry: Basics
High yield facts and clear explanation, the fundamental elements and efficient route to master biochem!
More detail
By Mia Y. on 03. April 2018 for Biochemistry: Basics
I am new to this curriculum, however, I would like the lectures to be longer. I am hopeful that other lectures are much more detailed, e.g. gene regulation/expression.
Lecturer is very knowledgeable, clearly.
I'm enjoying of the course
By Victor Rodrigo F. on 03. April 2018 for Biochemistry: Basics
I liked very much this course. It's very interesting and The good teachers.
An excellent coverage on Basics
By Timothy B. on 27. January 2018 for Biochemistry: Basics
How I wish I had this when I first started medical school. The explanations were clear and concise. The use of simple words enable me to understand it well as well as the option to pause at anytime allows further internalization of the facts.
Very good lecture
By Karan D. on 02. December 2017 for Biochemistry: Basics
Very good explanation of concepts. Animation could be added for excellent explanation.
Outstanding Lecture- Artistically Presented!
By Neuer N. on 28. November 2017 for History – Introduction to Biochemistry
The lecture was very intriguing! Dr. Ahern is so well articulated!
intro to biochemistry and how the biochemistry is related to our daily life
By Neuer N. on 05. November 2017 for Overview – Introduction to Biochemistry
the lecture is good it educates on how bichemistry is related to other fields in life
Concise and informative
By Iuliana V. on 27. October 2017 for Peptide Bonds – Peptides
The courses are very concisely presented, it is really useful for preping for an exam
More details
By Hanaa A. on 26. October 2017 for R-group Categories – Amino Acids
Need more details
Need to go slower
But overall not bad
good lecture
By Janes S. on 16. October 2017 for R-group Categories – Amino Acids
The lecturer is very good at getting biochemistry across.
Well explained and understandable up and until here.
All his teachings
By Schola A. on 04. October 2017 for Metabolism – Oxidation and Reduction in Metabolism
Professor Dr. Kevin Ahern is very clear in presenting.
Love the short but very informative lectures.
Would recommend to everyone, those with or without biochemistry understanding.
Thank you very much
Professor and Thanks to the maker of Lecturio.
Well done
By Fransiska H. on 27. September 2017 for Biochemistry: Basics
Everything was on point ..
Will definitely share with my friends.
Finally, some easily accessible and excellent lectures!
By Johan Fritz G. on 21. September 2017 for History – Introduction to Biochemistry
Very concisely put, I love the lectures, very helpful indeed. Now all I need to do is work my ass off to get the dollars to pay for this awesome lecture. Thank you Lecturio!
Wonderful course utilizing high-yield information for a relatively quick review
By Thomas R. on 18. September 2017 for Biochemistry: Basics
I found the lectures to be very informative and composed of high-yield information for a relatively quick review of the subject. The only reason I give 4* is because of the few technical problems with the course; but, in regard to the course material, Lecturio provides a wonderful review of Biochemistry with Dr. Ahern.
Table of AA
By Laurent J. on 10. September 2017 for Structure – Amino Acids
I like the way that the AA are presented by a table between essentiel and non essential.
I will use it for my memorizing process.
Nice and concise.
By Mal D. on 10. September 2017 for Overview – Introduction to Biochemistry
Great/clear explanation, definitely recommend to students. For those that complained, please describe what's needed to be explained, or just keep your pie hole closed. Being ambiguous is pointless.
I like the questions after the lecture!
By tatyana s. on 04. September 2017 for Biochemistry: Basics
I like the teaching style and material presented! It is clear and understandable. Thank you!
History Introduction to Biochemistry
By Neuer N. on 25. August 2017 for History – Introduction to Biochemistry
Kevin Ahren explained the part very well. I liked the way he explaining this part. I am from business back ground.
its more than expected
By Neuer N. on 22. August 2017 for Catalysis – Protein Functions
i like this lecture.
way of representing and explaining is quiet good.
Need some more details
By Bhavishya N. on 16. August 2017 for Overview – Introduction to Biochemistry
Very nice but there's some need of detailed explanation and then it would be perfect
Thank you! Better than any of my teachers at my university!
By Sara P. on 20. July 2017 for Saccharides – Simple Carbohydrates
Awesome job, I wish you were my teacher at my university! So clear!
excellent
By luiz s. on 19. June 2017 for Biochemistry: Basics
very good course, i wish i have money to pay the all course
Biochemistry
By Yada C. on 16. May 2017 for Biochemistry: Basics
These lectures are made simple for someone like me who is very vulnerable to biochemistry.
overall great
By neha a. on 12. April 2017 for Biochemistry: Basics
As it's my first lecture, I think I have to work a lot for getting great benefits from your great tutors. hence I had to give this rate. right now there are no such dislikes. well, thanks a lot for the kind assistance.
Two Thumbs Up
By Khalena K. on 04. April 2017 for Biochemistry: Basics
Great lecturers and thorough explanations for every subject. After watching the lectures, I actually understood and retained because his lectures are extremely well organized.
Helpful for Medical students
By Ratna P. on 21. March 2017 for Biochemistry: Basics
This is quite helpful. I am a pre-Medical student and I'm fully satisfied by the leacture. I'm able to cover all the syllabus. It's helping me to save time. I dont need all those notes n stuffs. Most important i can practise lots of questions! I'll recommend all Medical students to take this amazing leacture for once so that you'll be able to understand.
Thankyou @lecturio! ????
I HAD A HARD TIME WTH THE AMINO ACIDSTHE CHEMISTRY WAS DIFFICULT TO GRASP
By Anthony B. on 22. February 2017 for Biochemistry: Basics
A ERY INTERESTING COURSE ,BUT REQIURES A LLOT OF STUDY TIME TO UNDERSTAND THE AMINO ACID CHEMISTRY.
TO BE HONEST, I AM A RETIRED 85 YR OLD PHARMACIST TREYING TOEEP UP.
Biochemistry is great so far!
By Amy K. on 20. February 2017 for Biochemistry: Basics
I really like the pace and clarity of this lecturer in how he presents the material. It would be nice to have animated pictures in signaling and transport. I also wish there were sections covering enzymatic catalysis & kinetics. Over all, very good! Thank you for making these
Infinity chances
By Audrea L. on 17. January 2017 for Biochemistry: Basics
I rate this five stars because I like to go over it while I do other things and more than once.
Great!
By Anna H. on 17. January 2017 for Biochemistry: Basics
The overview of biochemistry is really helpful! The images are great, and the lecture is really high quality.
Good speaker
By Tammi F. on 07. January 2017 for Biochemistry: Basics
I was hoping for a little more details about the chemical makeup of the topic, however, I like how the entire video is layed out and described.
Very motivational!
By Berk T. on 21. November 2016 for Biochemistry: Basics
Watching these videos from Lecturio is very much like being in my school. The difference is Lecturio is more fun and it encourages us every time we want.
Professor Ahern sets the stage for Understanding Biochemistry
By Christopher D. on 30. October 2016 for Biochemistry: Basics
With this introductory course, you will be exposed to the basic aspects of Biochemistry. Professor Ahern emphasizes that an in-depth knowledge of basic biochemistry will aid you tremendously in understanding life processes on a macroscopic level. This one course alone was well worth the one month payment of 24$. Every other course on top of that was an attractive but inessential addition..."icing on the cake"!
Great lectures!
By David Z. on 19. October 2016 for Biochemistry: Basics
Professor Ahern is truly excellent! Amazing choice. He clearly explains concepts and shows sincere enthusiasm for the topics. You need to bring him back for lectures on amino acids and the urea cycle as well as the electron transport chain.
Just add explanations
By Omar M. on 18. September 2016 for Biochemistry: Basics
Very easy to follow, good flow and simply explained, exceptional professor! just wish the questions had explanations then I would give the videos a higher score .
cool
By Ikechukwu I. on 12. September 2016 for Biochemistry: Basics
it has helped me build my understanding of biochemistry. looking forward to more lectures
Great Course!
By Tamela E. on 15. August 2016 for Biochemistry: Basics
Love this course and Professor Ahern's enthusiasm keeps my attention. Being able to watch the videos over and over helps the lessons sink in. For the first time I'm enjoying biochemistry. Thank you for making these videos so affordable and easy to use.
Amazing
By Nwanze T. on 05. August 2016 for Biochemistry: Basics
Since is starting this biochemistry, it's help me to remember some of the basic concepts that I have forgotten
This is an amazing course!
By Rasha I. on 25. July 2016 for Biochemistry: Basics
This is an amazing course!
Prof Kevin has made every single details simple and clear. His deep knowledge and understanding of this subject is well reflected in his teaching style.
The materials he used, are very attractive which makes it easy to memorize.
I would definetly recommend this for both under and postgradustes students.
Great Biochem Course!
By Jesse R. on 15. July 2016 for Biochemistry: Basics
This course is well done. It's thorough. I'm reviewing things I've learned, and also moving on to more advanced material very soon. I wish I would have had this during undergrad.
The biochemiThe biochemistry is one of the best because the professor Ahern is a terrific docent who makes one love the lectures
By Ever L. on 02. July 2016 for Biochemistry: Basics
The biochemiThe biochemistry is one of the best because the professor Ahern is a terrific docent who makes one love the lectures