Playlist
Show Playlist
Hide Playlist
Buffer: Definition & Titration Curves
-
Slides 07 pHBuffers AcidBaseBalance GeneralPhysiology.pdf
-
Download Lecture Overview
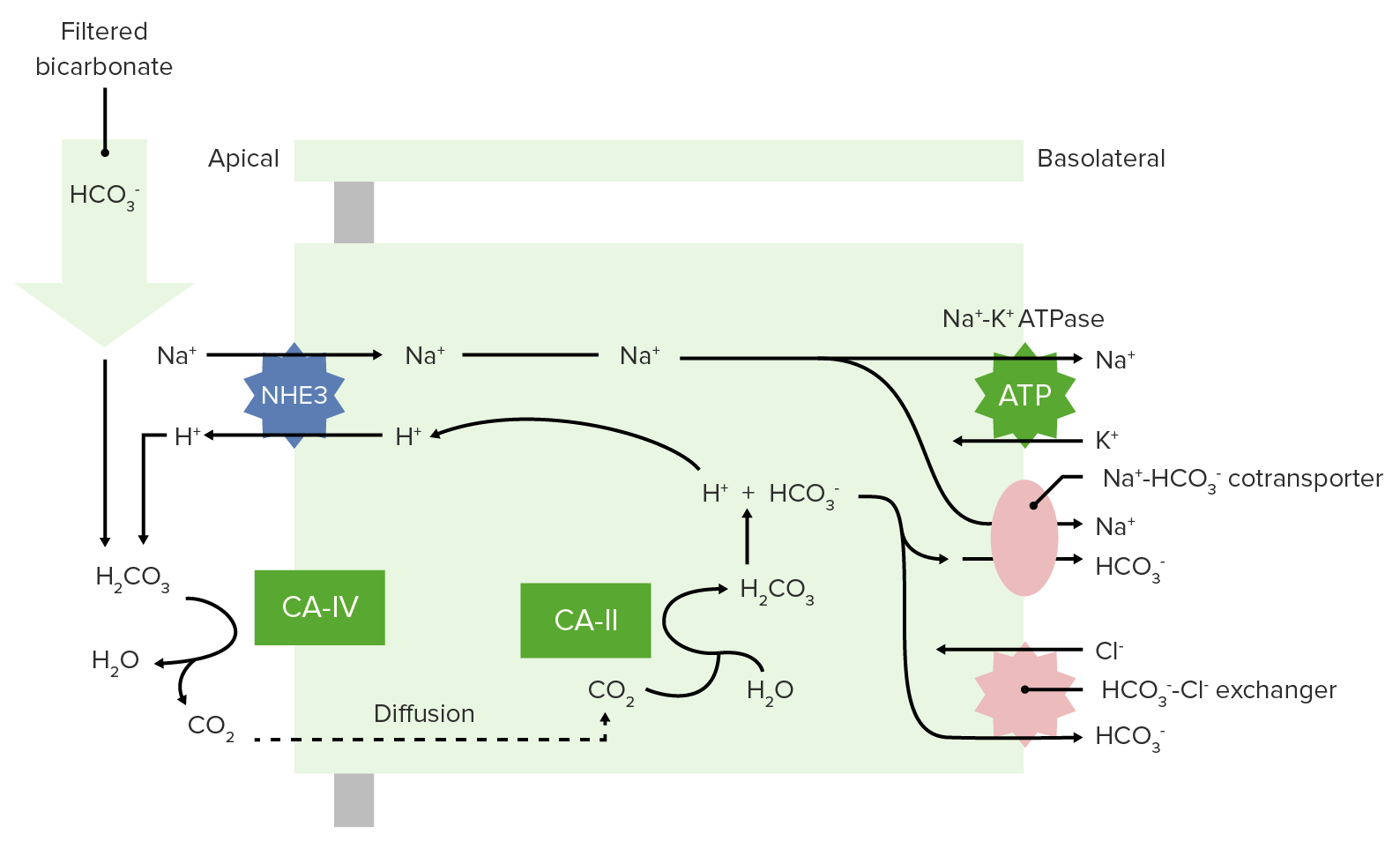
00:00 If we want to go through buffers and talk about them in more greater detail. 00:06 We have to spend a little bit of time going through, what the power of the buffer is? So different buffers will work at different pH’s. 00:17 and work at through a different level or amount of buffering capability. 00:23 This strict definition of a buffer is anything that consumes or releases a hydrogen ion (H+). 00:32 Again this helps to stabilizes pH. 00:35 The power of the buffer is related to the quantity of hydrogen ions it can remove. 00:43 So we often times talk about the blood is being a buffer itself. 00:47 And there are proteins in the blood that will help buffer pH changes. 00:53 Hemoglobin is one. 00:56 Other proteins can be these kind of buffers as well. 01:00 Cause remember a protein has a COON, so it can bind the hydrogen ion quite easily. 01:07 So the buffering power of whole blood is right around 25 millimole per unit of pH (~25mM/pH). 01:15 And this is even without bicarb present. 01:18 So we always have to account for the buffering capabilities of the blood, when we are discussing alkalemia’s and acidemia’s. 01:28 Now, let’s look at bicarbonate specifically. 01:32 So now we’re no longer talking about the buffering capability of whole blood. 01:36 If we just look at bicarb, we can develop what’s called a titration curve. 01:41 These titration curves are ways that we can deal with, how we change pH? and then in what form you’re going to have the buffer? Is it more likely going to be as a base or is it more likely going to be in a bound state? So, if we look at the curve, we have pH on the X-axis. 02:02 If we look at the other to Y-axis, it goes from zero to a hundred. And then from a hundred to zero. 02:10 These two changes allow us to develop the complete curve for this particular equation. 02:19 The blood is normally around 7,4. 02:23 The pK is a very important point. At this point, you have 50% that is in this HCO3- state. 02:32 and 50% that’s in the bound state which is the H2CO3. 02:38 This is the operating point in which you could go upwards or downwards. 02:43 And so we’re gonna find out the each individual buffer or titration curve that we look at will have a slightly different pK. 02:52 And we’ll talk through why the different pKs are important. 02:56 But for the blood and for bicarb, the pK in this case is 6,1. 03:03 These allows us to develop an equation to have a sol for what pH is it in the blood. 03:10 We can take 6,1, which is the pK, plus the log of the bicarb over the PCO2. 03:19 Now, PCO2 we have to also multiply it by a factor. 03:23 And that is to make sure it gets into solution that it’s solubility coefficient. 03:28 So you just take the bicarb divided by the PCO2. Take a log of that. add 6,1. 03:34 And that will be your pH.
About the Lecture
The lecture Buffer: Definition & Titration Curves by Thad Wilson, PhD is from the course Acid-Base Balance.
Included Quiz Questions
What is a buffer?
- A substance capable of releasing (or binding to) hydrogen ions.
- A substance present in the blood.
- A protein in the blood.
- A substance that binds to hydrogen and does not release it.
- A substance that disassociates in blood.
What is pKa?
- The pH at which the substance is 50% bound to H+ (H2CO3) and 50% free (HCO3-).
- The pH of the blood.
- The amount of carbonic acid in the blood.
- The amount of bicarbonate in the blood.
- The amount of CO2 in the blood.
How do we derive the pH of the blood when knowing the patient's serum bicarbonate and PCO2?
- We add the pKa of bicarbonate/carbonic acid to the log of the ratio between the patient's serum bicarbonate over serum PCO2x0.03 (CO2's solubility coefficient).
- We calculate the log of the ratio between the patient's serum bicarbonate over serum PCO2x0.03 (CO2's solubility coefficient).
- We calculate the log of the ratio between the patient's serum PCO2x0.03 (CO2's solubility coefficient) over serum bicarbonate.
- We add the pKa of bicarbonate/carbonic acid to the log of the ratio between the patient's serum PCO2x0.03 (CO2's solubility coefficient) over serum bicarbonate.
- We add the pH of the blood to the pKa of bicarbonate/carbonic acid.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
very good and excellent lecture.i like it.very good presentation