Playlist
Show Playlist
Hide Playlist
Bioavailability incl. Case Study – Elimination Kinetics
-
Slides Elimination Pharmacology.pdf
-
Download Lecture Overview
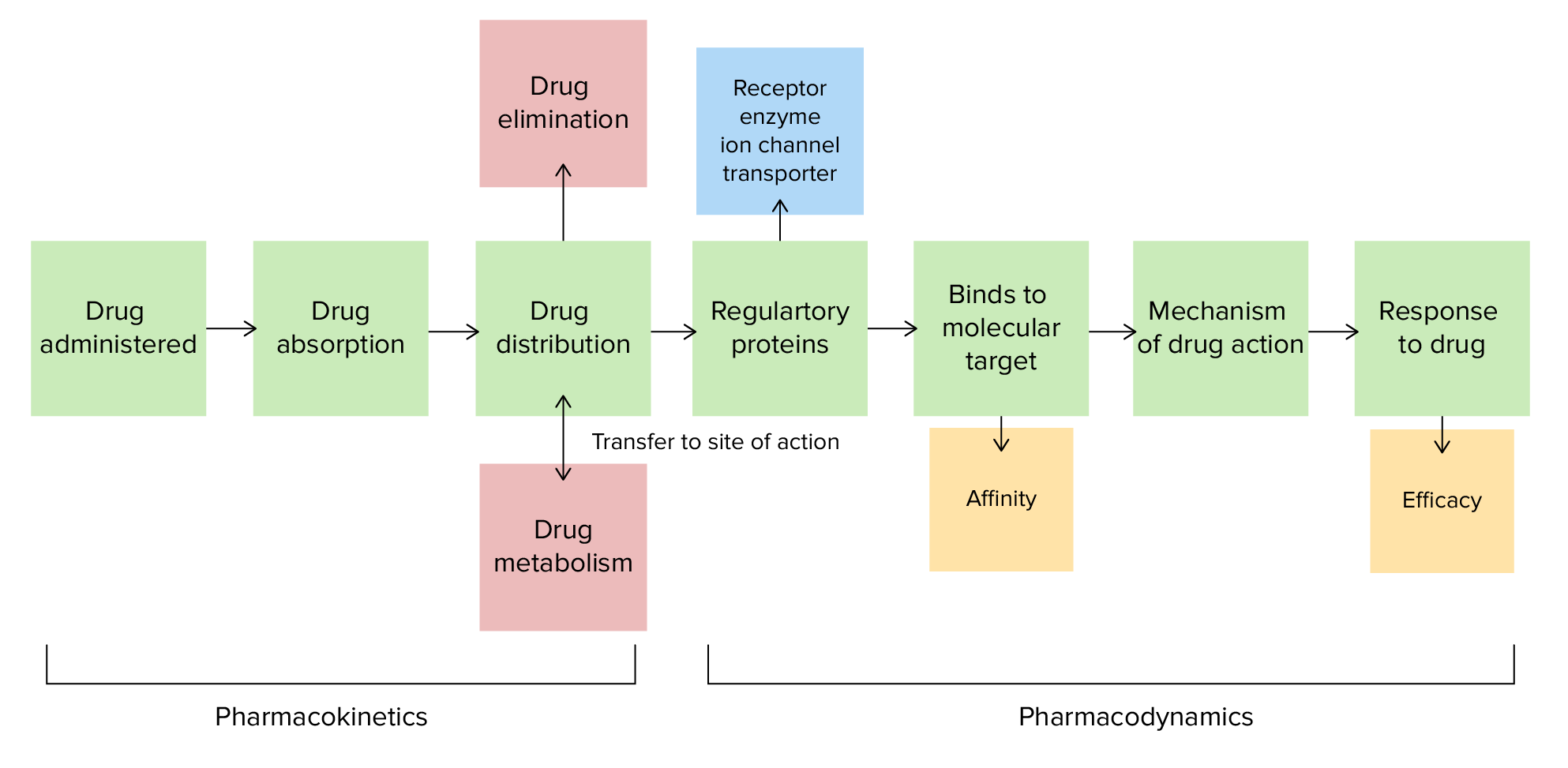
00:01 Let's do a question. Here is a common problem. Mr. Blackstar is in heart failure with a blood pressure of 190/70, and a heart rate of 122. He has got a respiratory rate of 18, which is a bit fast. 00:13 He was given 80 mg of furosemide orally to attempt diuresis, but he did not respond. 00:20 He was then given 20 mg intravenously and diuresed 3 litres. Why did this happen? Is it A, oral bioavailability is less than intravenous bioavailability? B, oral solubility is less than IV solubility? C, the volume of distribution for furosemide is high? Or D, the patient is allergic to oral furosemide? You're right, A. Oral bioavailability is less than intravenous bioavailablity. 00:56 Let's look at bioavailability as a concept. So you can see here, bioavailability represents the fraction of the dose that reaches the target circulation. So in the case of this particular drug, you can see that the intravenous drug has a very high initial concentration and goes down through first order kinetics. The oral drug also has first order kinetics, but it takes time to reach a certain concentration in the drug. Now the bioavailability is going to be proportional to how well you absorb the drug. In the case of IV, medication in this case is probably 100% absorbed. 01:39 It's proportional to first pass metabolism when it comes to the oral drug. So sometimes, you dump the drug into the bowel before it even gets to the circulation. 01:48 And finally, it's going to be proportional to your volume of distribution. 01:52 We measure also the amount of drug in the body using a concept called the area under the curve. 01:59 So, we take a look at the concentration curve and we measure the area underneath it, and that gives us a very good index in calculating bioavailablity of a drug. 02:09 Now, when we give multiple doses orally, the area under the curve is calculated using a graphical analysis program like this one, to calculate peak and trough levels.
About the Lecture
The lecture Bioavailability incl. Case Study – Elimination Kinetics by Pravin Shukle, MD is from the course Pharmacokinetics and Pharmacodynamics.
Included Quiz Questions
Which route of administration is most likely to have the highest bioavailability for a given drug?
- Intravenous
- Intramuscular
- Oral
- Rectal
- Sublingual
Drug X has a bioavailability of 90% when administered rectally, but only 40% when administered orally. Which statement best explains this discrepancy?
- The oral form undergoes first-pass metabolism.
- The rectally administered form is more readily absorbed.
- The oral form has a higher dose.
- The volume of distribution is lower when administered rectally.
- Rectal administration undergoes enterohepatic circulation.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |