Playlist
Show Playlist
Hide Playlist
Humoral Immunity: Antibody Polymers and Antibody Binding to Antigens
-
08 Slides Humoral Immunity.pdf
-
Download Lecture Overview
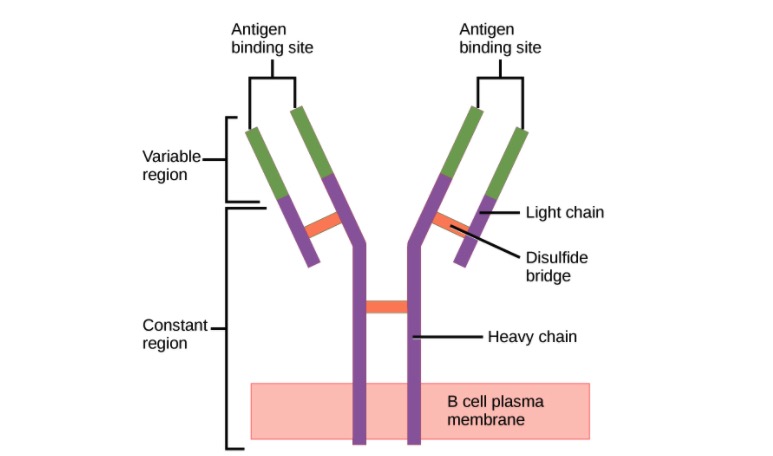
00:01 Two of the five classes of antibody can actually polymerize, form polymers. 00:06 Those two classes are IgA and IgM. 00:11 So you can find immunoglobulins as a single unit if you like, what immunologists refer to as a monomer. 00:20 And IgG, IgD, IgE and IgM when it’s found on the cell surface acting as the B-cell receptor of B-cells. 00:30 And IgA in the blood circulation. 00:33 These are all found in this monomeric form. 00:38 However IgA is also very dominant at mucosal surfaces. 00:44 And this so called secretory IgA is two individual units of IgA linked together by a molecule called the J chain. 00:54 There’s also an additional molecule called secretory component that is associated with this dimeric secretory IgA that is present at mucosal surfaces. 01:07 The IgM that is found in the circulation, in contrast to that found acting as the B-cell receptor on the surface of B-lymphocytes; when IgM is present in the circulation, five units of IgM link together to form a pentamer. 01:23 And again, this J chain molecule, it’s nothing to do with the J genes that you may have heard about when we were talking about immunoglobulin and T-cell receptor gene rearrangement. 01:34 This is a completely different thing, it’s a separate, it’s a protein molecule called J chain. 01:38 It joins together individual units of antibodies when they polymerize. 01:43 So for both secretory dimeric IgA and for circulating pentameric IgM, J chain facilitates that polymerization. 01:57 The different antibodies are not all present in the circulation at the same concentration. 02:03 In fact, far from it. 02:05 IgM is present in a concentration of around about 45 to 250 milligrams per deciliter (mg/dL) in the adult. 02:18 IgG is more dominant. 02:21 We have approximately 620 to 1400 mg/dL. 02:28 And in fact IgG is the most dominant class of antibody in the circulation. 02:33 Followed by IgA, which is present at around about 80 to 350 mg/dL. 02:41 And these three classes of antibody, the dominant IgG, second most concentrated IgA and then IgM. 02:51 They constitute the vast majority of antibody in the circulation. 02:55 Over 99% of your circulating antibody is either IgG, IgA or IgM. 03:02 Because we have vanishingly small amounts of IgD in the circulation, less than 8 mg/dL, and miniscule amounts of IgE. 03:15 When antibody binds to antigen, it recognizes particular amino acid sequences within the antigen, if the antigen is a protein antigen. 03:27 And these areas that are recognized by antibodies on protein, or indeed on other types of antigen, whether it’s a nucleic acid antigen or a lipid antigen, the area that is recognized by the antibody is referred to as the epitope. And the larger the antigen, the more epitopes will be present. So this single antigen may have several different epitopes, each one of which can be bound by a different antibody. So let’s just for the sake of argument say that this antigen is hen egg lysozyme, an antigen that lots of immunologists use in experiments. 04:08 So it may be that there’ll be one antibody against epitope 1 on the hen egg lysozyme, another against epitope 2 and so on. 04:18 So they’re all specific for the same antigen, but they’re different antibodies and they’re specific for different areas or different parts of that antigen; the different epitopes. 04:30 The binding of antibody to antigen involves non-covalent forces. 04:40 I’ve already mentioned that the two heavy chains are linked together by disulfide bonds, the light chains each one linked to a heavy chain by disulfide bonds. 04:48 So these are covalent bonds, they’re irreversible. 04:51 However the binding of antibody to antigen is a reversible process because all of the bonds that are involved are non-covalent. 05:02 So amino acids in the Complementarity Determining Regions interact with amino acids in the epitope using these non-covalent reversible forces. 05:12 And there are four major forces that are involved - hydrogen bonding, electrostatic interactions, Van de Waals interactions and hydrophobic interactions. 05:24 And the overall binding strength that is generated by these various types of non-covalent bonds results in the strength of binding of the antibody to the antigen, and we refer to that as the affinity of the antibody. 05:39 However, as we’ve already heard, antibodies have at least two antigen binding arms. 05:45 And in fact if you think about it, in secretory dimeric IgA there are four antigen binding arms. 05:51 And in circulating IgM, there are 10 antigen binding arms, because it’s five units of antibody linked together. 06:00 So the actual total strength of binding depends upon multivalent interactions that are occurring, and we refer to this as the functional affinity - the actual affinity that’s happening in practice, or the avidity of binding.
About the Lecture
The lecture Humoral Immunity: Antibody Polymers and Antibody Binding to Antigens by Peter Delves, PhD is from the course Humoral Immunity and Cell-mediated Immunity. It contains the following chapters:
- Antibody Polymers
- Antibody Binding to Antigens
Included Quiz Questions
Which of the following is the dominant type of immunoglobulin (Ig) in normal human serum?
- IgG
- IgM
- IgD
- IgA
- IgE
Which one of the following antibodies is NOT monomeric?
- Secretory IgA
- Cell surface IgM
- IgE
- IgD
- IgG
Which of the following immunoglobulins contains J (joining) chains?
- Circulating IgM
- IgE
- IgG
- Membrane IgM
- IgD
Which of the following is NOT a main force affecting the antibody-antigen reversible bond?
- Covalent bonds
- Electrostatic interactions
- Hydrogen bonds
- Van der Waals forces
- Hydrophobic interactions
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
These lectures are the most intresting Ive seen on immunology topics, delivered clearly, no mnemonics, pictures help significantly, seemed more exciting than any fiction. I would advise it to all who are preparing for the medical exams. I am just so grateful for finding this site.
Understood the concepts after not understanding them from reading my curriculum books..