Table of Contents

Image: “Molecular_and_Cell_Biology” by Kelvin Song. License: CC BY-SA 1.0
Introduction to Cellular Senescence and Stem Cell Aging
Aging is defined as a diminished response to stress, escalation of homeostatic imbalance and the enhanced threat from aging-related pathologies. With the advances in biomedical research, a number of theories proposed to expound aging have emerged and many are on their way. The most likely cause of aging is a multi-targeted attack on the normal functioning of the cell leading to disruption of the milieu interior of the cell and consequent detrimental effects which finally culminate in a diminution of a cell’s lifespan.
There is a multitude of diverse physiological traits described which characterize aging. The same can be tabulated as follows for easy memorization:
- Trait
- Stem cell exhaustion
- Mitochondrial dysfunction
- Cellular senescence
- Epigenetic alterations
- Loss of proteostasis
- Altered intercellular communication
- Telomere attrition
- Deregulated nutrient sensing
- Genomic instability
We now focus on cellular senescence and stem cell exhaustion and their role in aging.
Cellular Senescence
Overview
Cellular senescence implies irrevocable arrest of dividing cells. These cells possess grossly dysregulated phenotype, altered genotype, morphology and behavior distinct from their growth competent counterparts.
Different pathways
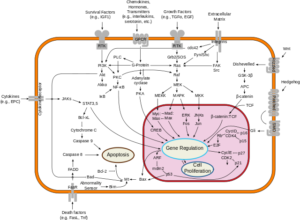
Image: “Signal Transduction Pathways” by Cybertory. License: CC BY-SA 3.0
Telomere attrition, Oxidative stress, and Oncogene activation are the different pathways which can induce cellular senescence.
Replicative senescence
Another important terminology closely related to cellular senescence is “Replicative senescence”. Telomere shortening occurs every time a cell divides. Once the telomere length reaches a critical threshold, it can no longer be protected by telomeric proteins.
This culminates in the exposure of DNA ends, subsequent DNA damage and ultimately cellular senescence. Thus, cellular senescence secondary to telomere attrition and DNA damage as a result of multiple cell divisions is known as “Replicative senescence”.
The key mechanisms by which senescent cells contribute to the diminution of the lifespan of a cell are enlisted as follows:
- Alteration of phenotypic behavior of surrounding cells
- Cellular dysfunction
- Reduction of growth-competent mitotic cells conglomerations
- Dysregulation of extracellular matrix and similar significant structural elements of the cell
- A propensity towards cancer formation
The biological impact of senescent cells at the molecular level is multi-pronged and can be summarized as follows:
- Up-regulation of growth factors
- Escalated levels of extracellular matrix (ECM)- degrading proteins
- Increased pro-inflammatory cytokines
- Up-regulation of matrix metalloproteinases (MMPs)
- Increased IL6, TNF α, Interleukin-8
- Abnormal secretion of growth factors
- Altered transcriptional profile
Cellular senescence has been incriminated in a number of disorders. The principal diseases associated with it are intervertebral disc degeneration, chronic hepatitis C, hepatocellular carcinoma, benign prostatic hyperplasia, type-II diabetes mellitus, a glomerular disease of the kidney and emphysema.
Clinical implications of Cellular Senescence and Stem Cell Aging
The concept of cellular senescence has deep far-reaching clinical consequences. Active, vigorous research concentrates on 3 key areas of cellular senescence, namely; prevention, removal, and replacement of senescent cells in tissues. Various therapies are taking shape in experimental setups already.
Telomerase therapy
The notion is to develop strategies to transiently turn on the telomerase activity in cells. This will limit telomere attrition and thus tame replicative senescence. However; not all cells are subject to this ideology. There exists a specific sub-population of cells which age independent of telomere shortening. Some are directly afflicted by DNA damage. These cells are unaffected by telomerase therapy.
Apoptosis-inducing pharmacotherapy
Cellular senescence is modulated by a fine balance maintained between the production of young cells from stem cells and removal of local damaged cells by scavenging and the immune system. The inspiration comes from the development of cell-surface marker specific anti-cancer drugs.
Immune system modulation
Rejuvenation therapy believes that accumulation of damaged senescent cells in tissues is predominantly a culmination of dysregulated and the aged immune system itself. There are various junctures in the immune system which, if reinstated, could curb the processes which accelerate aging.
The various important steps in the immune system affected by aging are:
- Regulation of dendritic cell function
- Recognition of proper target cell
- Display of accurate target cell-specific marker
- Responsive secondary activation of lymphocytes
Stem cell therapy
The deleterious effects of senescent cells are compounded by defective clearance of these cells associated with the diminished production of young healthy cells by a local niche of stem cells. Aging might itself decrease the vigor of the local stem cells. In these circumstances, stem cell rejuvenation therapy by the addition of stem cell populations into the tissues after removal of senescent cells is an interesting notion.
Stem Cell Exhaustion
Adult stem cells preservation is crucial for the maintenance of tissue homeostasis; especially as the cell grows older. Aging is associated with the attrition of stem cells. This is known as stem cell exhaustion. Exhaustion of the stem cell pool is brought in by a multitude of diverse processes such as dysregulated metabolic signaling, premature aging secondary to oncogenic insult to the somatic genome and other pathways which contribute to the decline in the functionality of the cell.
Adult stem cells regulate the key signaling pathways responsible for the emergence of age-related pathologies. Stem cell exhaustion leads to anemia, myelodysplasia, osteoporosis and decreased fracture repair, decreased repair of muscle fibers, decreased intestinal function and cancer. Highly advanced therapeutic manipulations of a stem cells niche to affect healthspan and lifespan of an individual is not a distant dream now.
Stem cell exhaustion is an integrative hallmark of aging, the culprit of the phenotype. It is an integrated consequence of so many others of these hallmarks. Age-related genomic instability and DNA damage are proposed to result in altered differentiation of stem cells which fail to provide optimum replacement of healthy cells and hence ultimately lead to decreased longevity of life.
Stem cells and the immune system are in the positive feedback loop when it comes to accelerating the aging phenotype. The genomic and transcriptomic profile of stem cells also undergoes changes as stem cell exhaustion occurs.
Stem cell and immune system
Stem cell exhaustion and dysregulated aged immune system are in a vicious cycle with positive feedback mechanism linking the two. Thus, while the altered function of stem cells leads to the further dysfunctional immune system, the defunct immune system creates a pro-inflammatory aging milieu which further hampers the functionality of the stem cells. This vicious cycle is thought to culminate in accelerated senescent phenotype.
Altered gene expression profile in stem cell exhaustion
Network analysis studies have revealed differential regulation of the different set of genes in adult stem cells as they are subjected to exhaustion and subsequently cellular senescence. Studies on aging from the cytogenetic perspective reveal a fine balance between proto-oncogenes and tumor suppressor genes, each pushing the growth curve in opposite directions. Aging can be looked upon as a disruption of this fine balance regulating alternative cellular states.
The key genes involved are as follows:
| Proto-oncogenes: | BMI 1 Wnt/β-catenin |
| Tumor suppressor genes: | INK4 ARF AIMP3/p18 |
The principal changes which occur at the molecular level to bring about this altered genomic and transcriptomic profiling can be summarized as follows:
- Up-regulation of glycan metabolism
- Slower DNA unwinding
- Dysfunctional regulators of senescence
- Perturbation of germline genetic heterogeneity
- Increased collagen cross-linking
- Capillary basement membrane thickening
- Overexpression of pro-inflammatory genes and inflammatory adipocytokines; especially in females
- Aberrant insulin-IGF1, FoxO and mTOR signaling, glucose and fatty acid metabolism
- Overexpression of pro-inflammatory genes; especially in females
- Decreased Na+K+ ATPase activity
Stem cells and Metabolic stress
Adult stem cells are victimized by a number of metabolic stressful insults. These stressors challenge the reparative and regenerative potential of the stem cells. Stem cell exhaustion secondary to these stressful circumstances results in aging. The key metabolic stressors can be summarized as follows:
- Oxidative stress
- Nutrient deprivation
- Abnormal proteome; especially involving proteins in cytoskeletal organization and anti-oxidant defense armamentarium
- Altered protein infrastructure dealing with the redox status of the cell
The critical cellular pathways implicated in stem cell exhaustion and aging can be summarized as follows:
Explanation of Wnt signaling pathway
The canonical Wnt signaling pathway is crucial in the fate determination of stem cells. Stem cell exhaustion is regulated in a disciplined manner by Wnt pathway through extensive crosstalk with multiple other key regulatory pathways of the cell such as the SMAD signaling pathway; BMP, NOTCH, and prostaglandin E2 pathways.
The following are modulated by the Wnt signaling pathway:
- Regulation of stem cell proliferation
- Self-renewal
- Differentiation
- Embryonic patterning
- Stem cell maintenance
- Stem cell senescence
The Klotho mouse model of aging points to aberrant Wnt signaling as the culprit pathway in stem cell exhaustion and premature senescence of stem cells with decreased self-renewal and early senescent phenotype. There is evidence to correlate gradual accumulation of Progerin and diminishing Klotho levels with human aging.
Explanation of FoxO family
The FoxO transcription factor signaling pathway regulates the cellular response to oxidative stress. It coordinates the composite response of a cell to tumor suppression, metabolism, and longevity of lifespan of a cell. It regulates multiple intersecting cellular pathways and affects a multitude of key fundamental cell functions, such as the following:
- Stress resistance
- Gluconeogenesis
- Cell cycle arrest
- Autophagy
- Apoptosis
- Neuropeptide secretion
- Atrophy
Bestowed with ubiquitous expression, FoxO functions are still highly specialized and customized uniquely to distinct cell types. Redirection of β-catenin function as a result of oxidative stress-mediated through FoxO family of transcription factors culminates in the altered transcriptomic profile of stem cells and affects cellular homeostasis and cellular senescence. FoxO transcription factors also play a role in stem cell exhaustion by altering the structural and functional integrity of the aging immune system.
Clinical implications
Extensive understanding of the role of stem cell exhaustion in aging has triggered a lot of fascination and vigor in interventional regenerative medicine. Engineering cells and tissues for replacement and regeneration of diseased and injured cellular entities, such as in neuromuscular systems, can confer resistance to aging.
Ex vivo aging of stem cells in rodent cells is an upcoming area of research. Partial reprogramming involves moving adult stem cells from limited multi-potency towards pluripotency by altering the transcriptional phenotype of stem cells. This approach can be used to preserve the proliferative potential and regenerative capacity of the stem cells; thus evading exhaustion.
Summary of Cellular Senescence and Stem Cell Aging
Aging is defined as the diminished response to stress, escalation of homeostatic imbalance and the enhanced threat from aging-related pathologies. There is a multitude of diverse physiological traits described which characterize aging.
Cellular senescence implies irrevocable arrest of dividing cells. These cells possess grossly dysregulated phenotype, altered genotype, morphology and behavior distinct from their growth competent counterparts. Cellular senescence is an antagonistic hallmark of aging; a response to damage.
There are different pathways which can induce cellular senescence. Cellular senescence secondary to telomere attrition and DNA damage as a result of multiple cell divisions is known as “Replicative senescence”. Cellular senescence has been incriminated in a number of disorders.
The concept of cellular senescence has deep far-reaching clinical consequences. It opens avenues for therapeutic manipulations such as telomerase therapy, apoptosis-inducing pharmacotherapy, stem cell therapy, and immune system modulation. Adult stem cells preservation is crucial for the maintenance of tissue homeostasis; especially as the cell grows older. Aging is associated with the attrition of stem cells. This is known as stem cell exhaustion.
Stem cell exhaustion leads to anemia, myelodysplasia, osteoporosis and decreased fracture repair, decreased repair of muscle fibers, decreased intestinal function and cancer.
Stem cell exhaustion is an integrative hallmark of aging. It is influenced by multiple factors, such as oxidative stress, metabolic stress, dysregulated immune system, altered genomic and transcriptomic profiling, disrupted balance between proto-oncogenes and tumor suppressor genes. Wnt signaling pathway and FoxO transcription factors are key regulators of stem cell exhaustion.
Ex vivo aging of stem cells, partial reprogramming, and tissue engineering are potential areas of research in relation to stem cell exhaustion.
A summary of the hallmarks of cellular aging
| Primary hallmarks (Causes of damage) | Antagonistic hallmarks (Response to damage) | Integrative hallmarks (Culprits of the phenotype) |
| Genomic instability | Deregulated nutrient sensing | Stem cell exhausting |
| Telomere attrition | Mitochondrial dysfunction | |
| Epigenetic alterations | Cellular senescence | Altered intracellular communication |
| Loss of proteostasis |